Abstract
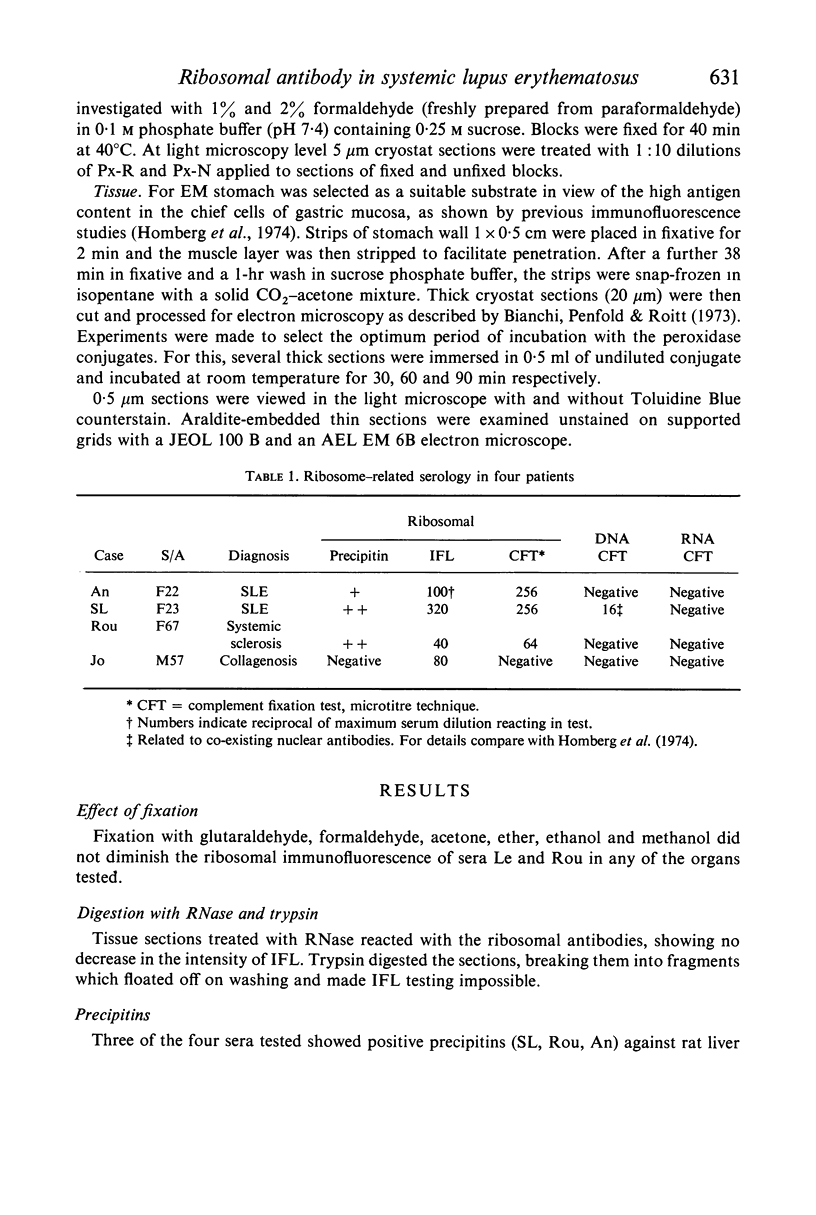
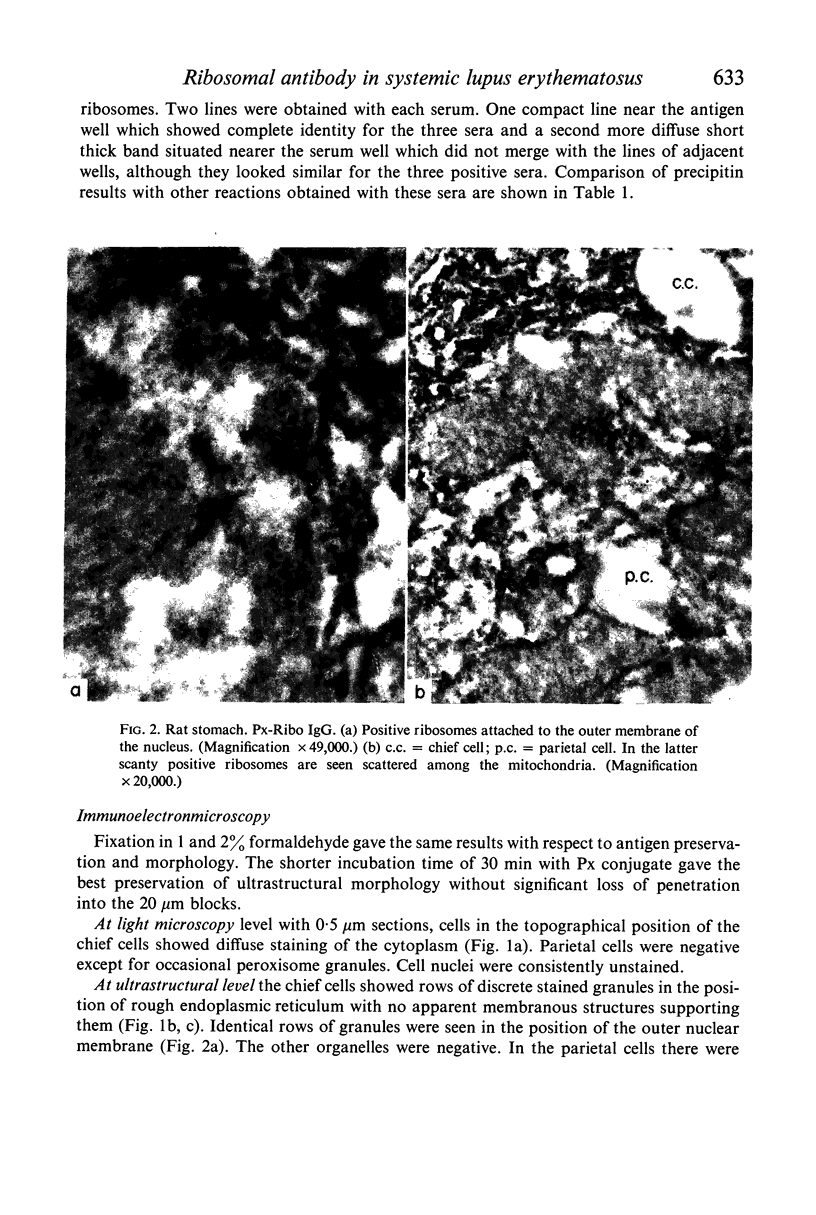
The ribosomal antibody detected by tissue immunofluorescence in about 1% of SLE patients was conjugated with peroxidase and its antigen localized by immunoelectronmicroscopy, using rat stomach as substrate. The antibody stained ribosomes on rough ER, single ribosomes and polyribosomes, but not membranes. Gastric chief cells reacted most intensely; ribosomes in plasma cells, lymphocytes and eosinophils seen between gastric cells were also positive, thus confirming earlier immunofluorescence studies which showed that all tissues react in relation to their ribosomal content. Nucleolar ribosomes were unreactive.
The ribosomal antigen was resistant to glutaraldehyde, formaldehyde, acetone, ether, ethanol, methanol and detergents such as deoxycholate. Digestion of the sections with RNase did not diminish the immunofluorescence. Trypsin could not be used on sections but is known from previous CFT studies to destroy the ribosomal antigen. It was concluded that this antigen is a ribosomal protein unlike other tissue autoantigens of which several studied so far are lipoprotein in nature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora D. J., de Lamirande G. Ribonuclease activity in the hepatic ribonucleoprotein particles of growing rat. Arch Biochem Biophys. 1968 Feb;123(2):416–417. doi: 10.1016/0003-9861(68)90153-7. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- BAUR S., ROITT I. M., DONIACH D. CHARACTERIZATION OF THE HUMAN GASTRIC PARIETAL CELL AUTO-ANTIGEN. Immunology. 1965 Jan;8:62–68. [PMC free article] [PubMed] [Google Scholar]
- Ben-Yoseph Y., Shapira E., Doniach D. Further purification of the mitochondrial inner membrane autoantigen reacting with primary biliary cirrhosis sera. Immunology. 1974 Feb;26(2):311–321. [PMC free article] [PubMed] [Google Scholar]
- Berg P. A., Roitt I. M., Doniach D., Horne R. W. Mitochondrial antibodies in primary biliary cirrhosis. 3. Characterization of the inner-membrane complement fixing antigen. Clin Exp Immunol. 1969 May;4(5):511–525. [PMC free article] [PubMed] [Google Scholar]
- Bianchi F. B., Penfold P. L., Roitt I. M. Mitochondrial antibodies in primary biliary cirrhosis. V. Ultrastructural localization of the antigen to the inner mitochondrial membrane using a direct peroxidase conjugate. Br J Exp Pathol. 1973 Dec;54(6):652–657. [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie R. B., McDonald E., Anderson J. R., Gray K. Immunological features of idiopathic Addison's disease: characterization of the adrenocortical antigens. Clin Exp Immunol. 1968 Feb;3(2):119–131. [PMC free article] [PubMed] [Google Scholar]
- Hopf U., Meyer zum Büschenfelde K. H., Freudenberg J. Liver-specific antigens of different species. II. Localization of a membrane antigen at cell surface of isolated hepatocytes. Clin Exp Immunol. 1974 Jan;16(1):117–123. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROITT I. M., LING N. R., DONIACH D., COUCHMAN K. G. THE CYTOPLASMIC AUTO-ANTIGEN OF THE HUMAN THYROID. I. IMMUNOLOGICAL AND BIOCHEMICAL CHARACTERISTICS. Immunology. 1964 Jul;7:375–393. [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Bianchi F. B., Doniach D. Characterization of the microsomal antigen related to a subclass of active chronic hepatitis. Immunology. 1974 Mar;26(3):589–601. [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Swana G., Doniach D. Microsomal antibodies in active chronic hepatitis and other disorders. Clin Exp Immunol. 1973 Nov;15(3):331–344. [PMC free article] [PubMed] [Google Scholar]
- Sturgill B. C., Preble M. R. Antibody to ribosomes in systemic lupus erythematosus: demonstration by immunofluorescence and precipitation in agar. Arthritis Rheum. 1967 Dec;10(6):538–543. doi: 10.1002/art.1780100606. [DOI] [PubMed] [Google Scholar]