Abstract
Recently, we identified a GTPase-activating protein for the ADP ribosylation factor family of small GTP-binding proteins that we call GIT1. This protein initially was identified as an interacting partner for the G protein-coupled receptor kinases, and its overexpression was found to affect signaling and internalization of the prototypical β2-adrenergic receptor. Here, we report that GIT1 overexpression regulates internalization of numerous, but not all, G protein-coupled receptors. The specificity of the GIT1 effect is not related to the type of G protein to which a receptor couples, but rather to the endocytic route it uses. GIT1 only affects the function of G protein-coupled receptors that are internalized through the clathrin-coated pit pathway in a β-arrestin- and dynamin-sensitive manner. Furthermore, the GIT1 effect is not limited to G protein-coupled receptors because overexpression of this protein also affects internalization of the epidermal growth factor receptor. However, constitutive agonist-independent internalization is not regulated by GIT1, because transferrin uptake is not affected by GIT1 overexpression. Thus, GIT1 is a protein involved in regulating the function of signaling receptors internalized through the clathrin pathway and can be used as a diagnostic tool for defining the endocytic pathway of a receptor.
G protein-coupled receptor function is tightly regulated by numerous downstream signaling events. Agonist stimulation of a G protein-coupled receptor triggers a conformational change allowing activation of coupled G proteins through GDP–GTP exchange (1). This conformational change also promotes activation of the G protein-coupled receptor kinases (GRKs) to phosphorylate the activated receptor, allowing binding of β-arrestin proteins that sterically prevent further coupling to heterotrimeric G proteins (2, 3). Binding of β-arrestins to GRK-phosphorylated G protein-coupled receptors also is believed to initiate receptor sequestration into endosomal recycling compartments (4). This event appears to be one mechanism by which activated receptors are dephosphorylated and resensitized (3, 5, 6). We recently identified a GRK-interacting protein that we call GIT1 (GRK-interactor 1) and showed that overexpression of this protein in HEK 293 cells markedly affects signaling and trafficking of the β2-adrenergic receptor (β2AR) (7). Interestingly, GIT1 contains an active ADP ribosylation factor (ARF) GTPase-activating protein domain (GAP) at its amino terminus and binds GRKs through a region located near the carboxyl terminus. The ability of GIT1 to inhibit β2AR internalization requires the intact ARF GAP domain, suggesting that GTP–GDP cycling of ARF proteins may be involved in this process (7). Further, there appear to be at least three members of the GIT protein family, GIT1, GIT2/CAT2, and PKL (7–9), which also interact with the PIX/PAK complex and with paxillin, as well as PIP3 lipids (R.T.P., unpublished observations; and N. Vitale, personal communication) (8, 9). The functional consequences of these interactions for receptor biology still remain to be defined.
In our first report (7), we noted that GIT1 overexpression inhibited β2AR internalization. Sequestration of cell surface receptors can occur through different pathways, which differ in the size and the composition of the proto-vesicle coat (clathrin, nonclathrin, caveolae, and macropinosome) (10). The most extensively studied mechanism for receptor-mediated endocytosis occurs by means of the clathrin-coated pits and vesicles (11). Proteins like the arrestins and dynamins play important roles in the function of clathrin-coated pits. Arrestins have been shown to interact with clathrin (12–14), the major protein component of clathrin-coated pits, as well as with the clathrin adaptor protein AP-2 (15), and the N-ethylmaleimide-sensitive fusion protein (NSF) (16). Furthermore, dynamin, a large GTPase, is involved in pinching off of clathrin-coated vesicles from the plasma membrane (17–22). Moreover, dynamin also has been localized in caveolae, and its GTPase activity also appears to be essential for budding of vesicles from these structures (23, 24).
Although many studies have addressed the precise functions of proteins that mediate the complex process of clathrin-dependent internalization, additional factors that are required for this and other endocytic pathways remain to be defined. In an attempt to further delineate the role of GIT1 in regulating cell surface receptor function and trafficking, we examined the role of this newly appreciated GRK-interacting protein in the internalization of a variety of receptors.
Materials and Methods
Materials.
Tissue culture media and FBS were obtained from Life Technologies (Rockville, MD). Isoproterenol, angiotensin II, anti-Flag M2, and anti-c-myc mAbs, anti-mouse IgG-FITC-conjugated antibody, and monodansylcadaverine were purchased from Sigma. Vasoactive intestinal peptide and endothelin-1 were purchased from Peninsula Laboratories. Mouse anti-hemagglutinin (HA) 12CA5 mAb was obtained from Roche Molecular Biochemicals. Fluorescein-labeled transferrin and rhodamine-labeled goat anti-mouse antibodies were from Molecular Probes. Recombinant epidermal growth factor (EGF) was obtained from Calbiochem. Anti-human EGF receptor (clone 528) antibody was obtained from Santa Cruz Biotechnology.
Plasmids.
The rat vasoactive intestinal peptide (VIP)1 receptor (25) was amplified from rat liver cDNA library and subcloned into the pcDNA1/Amp vector (Invitrogen). The pcDNA1/Amp-Flag-VIP1 receptor was generated by amplifying the entire receptor cDNA using an oligonucleotide primer that inserted a HA signal sequence and Flag epitope (DYKDDDDA) immediately before amino acid 1 of the mature receptor, replacing the endogenous signal sequence, as described for the β2AR (26). The pBK-Flag-A2B was prepared in the same manner from the corresponding human wild-type receptor cDNA. The pRK5-HA-M1 and pRK5-HA-M2 muscarinic acetylcholine receptors were generated by amplifying the receptor cDNAs using oligonucleotide primers that inserted HA signal sequence and HA epitope (YPYDVPDYA, recognized by the 12CA5 mAb) immediately after the initiator methionine. All constructs were verified by sequencing. The pBK-Flag-β2AR (7), pBK-GIT1-Flag (7), pcDNA3-Flag-β1 adrenergic receptor (27), pcDNA3-HA-μ-opioid receptor (28), pcDNA1-Flag-endothelin B receptor (29), and pcDNA1-HA-angiotensin 1A receptor (30) have been described.
Cell Culture and Transfection.
HEK 293 cells were maintained in MEM supplemented with 10% FBS, 100 units/ml penicillin G, and 100 μg/ml streptomycin sulfate at 37°C, 5% CO2. Before transfection, cells were grown to 60–75% confluency. Cells were transiently transfected overnight in 100-mm dishes (Falcon) with 12 μg of total plasmid DNA using calcium phosphate precipitation (31). After transfection (≈16 h), the cells were incubated with fresh medium and allowed to recover for 8 h before being reseeded into 12- or 6-well dishes. The generation of the GIT1 stable cell line has been described (7).
Sequestration Assays.
Receptor sequestration was determined by flow cytometry as described (32) using Flag-β2AR, Flag-β1AR, Flag-VIP1R, Flag A2BR, HA-μOR, Flag-ETB, HA-M1 MR, HA-M2 MR, HA-AT1AR, and EGFR transiently transfected in HEK 293 cells or in cells stably overexpressing GIT1. Receptor sequestration was defined as the fraction of cell surface receptor of naive cells that was no longer accessible to antibodies outside the cells after agonist treatment. Baseline fluorescence from cells transfected with empty vector was subtracted from each sample. In some experiments, cells were treated with monodansylcadaverine (400 μM) for 30 min before agonist stimulation.
Transferrin Uptake Assay.
Transferrin uptake assays were performed as described (33). Briefly, GIT1-Flag and dynamin II K44A were transfected into COS 7 cells on coverslips using Lipofectamine (GIBCO/BRL) according to the manufacturer's instructions. Forty-eight hours after transfection, cells were serum-starved for 1 h, labeled for 15 min with 20 μg/ml of fluorescein-labeled transferrin, rinsed, and fixed with 3% paraformaldehyde for 25 min. The cells then were permeabilized for 5 min with acetone (−20°C) and immunostained with anti-Flag or anti-c-myc antibodies and rhodamine-labeled anti-mouse IgG secondary antibodies. Digital fluorescence images were acquired with a Photometrics (Tuscon, AZ) cooled charge-coupled device mounted on a Zeiss Axiovert 135 epifluorescence microscope.
Data Analysis.
The mean and SEM are expressed for values obtained from the number of separate experiments indicated. Statistical analysis was performed by a two-way ANOVA followed by an unpaired Student's t test.
Results
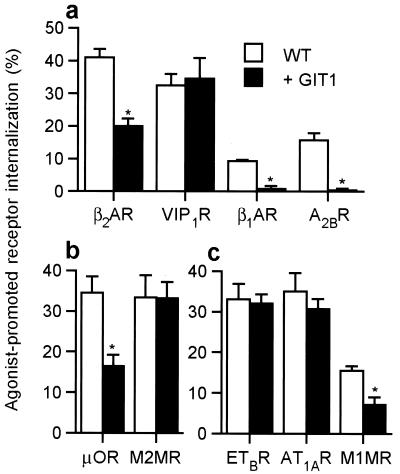
We recently demonstrated that GIT1 overexpression in HEK 293 cells leads to a marked reduction in cAMP signaling ability of the endogenous β2AR along with increased receptor phosphorylation and reduced agonist-dependent receptor sequestration from the cell surface (7). We proposed that GIT1 overexpression inhibits agonist-promoted internalization of the β2AR, which in turn leads to accumulation of phosphorylated receptors and reduced signaling capacity. To explore the generality of this receptor modulatory activity of GIT1, we first examined the ability of GIT1 overexpression to inhibit the agonist-promoted internalization of other Gs-coupled receptors. Using transient expression of Flag or HA epitope-tagged receptor cDNAs, cell surface receptor number was assessed before and after a 30-min agonist stimulation (Fig. 1a). Similar to the β2AR, the agonist-stimulated internalization of the adenosine 2B and β1-adrenergic receptors was markedly reduced in GIT1-overexpressing cells. In contrast, the agonist-promoted endocytosis of the VIP1 receptor was not affected by GIT1 overexpression (Fig. 1a). Furthermore, stable overexpression of GIT1 reduced cAMP production stimulated by activated endogenously expressed adenosine 2B receptor, as well as the β2AR, but not by the VIP1 receptor (data not shown). Taken together, these results provide strong evidence that the inhibitory action of GIT1 overexpression on receptor function is not specific to the β2AR, but neither is it common to all Gs-coupled receptors.
Figure 1.
Effect of GIT1 overexpression on internalization of G protein-coupled receptors. (a) GIT1 overexpression affects the internalization of some Gs-coupled receptors. Wild-type (WT) and GIT1-overexpressing HEK293 cells transiently transfected with Flag-tagged β2AR, β1AR, Flag-VIP1R, or A2BR were incubated with isoproterenol (10 μM), VIP (0.1 μM), or adenosine (10 μM), respectively, for 30 min before incubation with antibodies as described in Materials and Methods. (b) Internalization of the Gi-coupled HA-μOR and HA-M2 MR was assessed in WT and GIT1-overexpressing 293 cells after a 30-min stimulation with etorphine (500 nM) or acetylcholine (ACh) (100 μM), respectively. (c) Internalization of the Gq-coupled Flag-ETBR, the HA-AT1AR, and HA-M1 MR was measured in WT and GIT1 overexpressing 293 cells after a 30-min stimulation with endothelin-1 (0.1 μM), angiotensin II (1 μM), or ACh (100 μM), respectively. Results are means ± SEM for 3–6 independent experiments done in duplicate. *, P < 0.001.
We next tested the ability of GIT1 overexpression to alter endocytosis of receptors coupled to distinct G proteins. We chose the Gi-coupled μ-opioid receptor (μOR) and M2 muscarinic receptor (MR), and the Gq-coupled M1 MR, endothelin B receptor (ETBR), and angiotensin 1A receptor (AT1AR). All five receptors underwent substantial agonist-promoted sequestration from the cell surface (Fig. 1 b and c). Agonist-dependent internalization of the μOR and M1 MR was inhibited by GIT1 overexpression. However, agonist-stimulated endocytosis of the M2 MR, ETBR, and AT1AR was not affected by GIT1. These results show that the ability of GIT1 to inhibit receptor endocytosis does not correlate with the type of G protein to which a receptor couples, because at least some Gs-, Gi-, and Gq-coupled receptors can be regulated by GIT1 overexpression, whereas others are not.
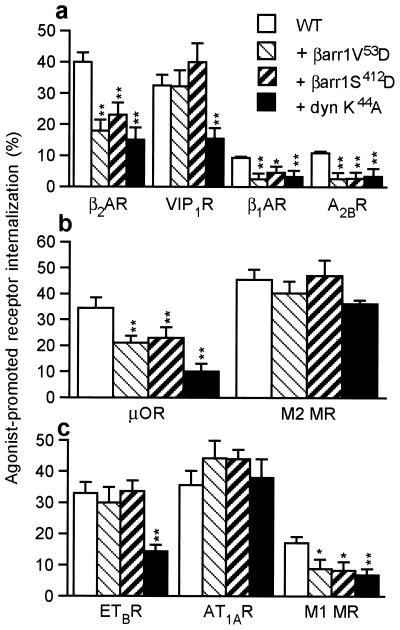
Studies of endocytosis of heptahelical receptors have demonstrated that several distinct pathways of sequestration from the cell surface exist. These pathways differ in their sensitivity to various inhibitors, such as mutants of the β-arrestin1 protein, β-arrestin 1 V53D, and S412D (14, 34, 35) or a mutant of dynamin defective in GTP binding (36, 37), dynamin I K44A. Because it has been demonstrated previously that agonist-promoted sequestration of the β2AR (12, 38), M1 MR (39, 40), and μOR (28, 41) is inhibited by both β-arrestin and dynamin-dominant negatives and that endocytosis of the M2 MR (42) and AT1AR (38) is insensitive to these inhibitors, we set out to test whether sensitivity to GIT1 might be correlated with sensitivity to these mutants. Thus, we next examined the ability of β-arrestin 1 V53D, β-arrestin 1 S412D, and dynamin I K44A to affect internalization of several heptahelical receptors. Of the five receptors affected by GIT1 overexpression, β2AR, β1AR, A2BR, μOR, and M1 MR, all five also were inhibited by both β-arrestin and dynamin-dominant negative mutants (Fig. 2). Of the four receptors unaffected by GIT1, VIP1R, ETBR, M2 MR, and AT1AR, all were unaffected by the two β-arrestin mutants (Fig. 2). Interestingly, the sequestration of the VIP1R and the ETBR was substantially inhibited by dynamin 1 K44A, whereas that of the M2 MR and AT1AR was not (Fig. 2). Thus, sensitivity to GIT1 appears correlated with sensitivity to β-arrestin mutants. However, sensitivity to dynamin K44A, while occurring for all GIT1-sensitive receptors, appears to be a feature of a wider class of G protein-coupled receptors (Table 1).
Figure 2.
Effect of β-arrestin and dynamin mutants on agonist-mediated internalization of G protein-coupled receptors. Sensitivity to β-arrestin 1 (βarr) V53D, β-arrestin 1 S412D, and dynamin (dyn) K44A is shown after a 30-min agonist stimulation. (a) Effect of dominant-negative mutants on internalization of Gs-coupled receptors. The Flag-β2AR and Flag-β1AR were stimulated by isoproterenol (10 μM), the Flag-VIP1R was stimulated by VIP (0.1 μM), and the Flag-A2BR was stimulated by adenosine (1 μM) for 30 min. (b) The Gi-coupled HA-μOR and HA-M2 MR were stimulated with etorphine (500 nM) or acetylcholine (ACh) (100 μM) for 30 min. (c) Effect of dominant-negative mutants on internalization of Gq-coupled receptors. The Flag-ETBR was stimulated with endothelin-1 (0.1 μM), the HA-AT1AR was stimulated with angiotensin II (0.1 μM), and the HA-M2 MR was stimulated with ACh (100 μM). Data represent the means ± SEM of 3–5 independent experiments. *, P < 0.01; **, P < 0.001.
Table 1.
Distinct patterns of inhibitor sensitivity of G protein-coupled receptor internalization pathways
| Pathway | Receptor | Agonist-stimulated internalization | GIT1- sensitivity | β-arrestin-mutant sensitivity | Dynamin-mutant sensitivity |
|---|---|---|---|---|---|
| I | β2AR | Yes | + | + | + |
| I | β1AR | Yes | + | + | + |
| I | A2BR | Yes | + | + | + |
| I | μOR | Yes | + | + | + |
| I | M1 MR | Yes | + | + | + |
| II | VIP1R | Yes | − | − | + |
| II | ETBR | Yes | − | − | + |
| III | AT1AR | Yes | − | − | − |
| III | M2 MR | Yes | − | − | − |
Receptors are grouped into four different pathways of internalization (I–III) according to their sensitivity to overexpression of GIT1 and mutants of β-arrestin and dynamin.
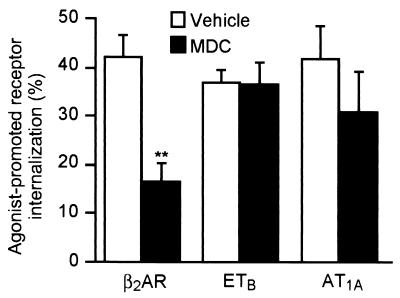
The sequestration of the β2AR has been reported previously to use the clathrin-coated pit pathway (4, 12, 43), suggesting that GIT1 sensitivity may correlate with receptor sequestration by means of a β-arrestin- and dynamin-sensitive clathrin-coated pit pathway. As a first step to show that the GIT1-sensitive sequestration pathway is indeed the clathrin-coated pit pathway, we examined receptor sequestration in cells treated with 400 μM monodansylcadaverine (MDC), which has been reported to stabilize clathrin cage assembly, thus inhibiting internalization (44). We tested the effect of MDC treatment on one representative receptor from each of the sequestration classes: namely, the β2AR (sensitive to GIT1, and mutants of β-arrestin and dynamin), the ETBR (sensitive to the dynamin mutant, but not GIT1 or mutants of β-arrestin), and the AT1AR (insensitive to GIT1 or mutants of β-arrestin and dynamin). A 30-min pretreatment of the cells with MDC substantially inhibited agonist-promoted sequestration of the β2AR, but was ineffective in inhibiting agonist-stimulated sequestration of either the ETBR or AT1AR (Fig. 3). These data suggest that the pattern of inhibition defined by sensitivity to GIT1, β-arrestin mutants, and the dynamin mutant may indeed fingerprint the clathrin-dependent pathway for receptor internalization.
Figure 3.
Effect of MDC treatment on internalization of the model G protein-coupled receptors. HEK 293 cells transiently transfected with Flag-β2AR, Flag-ETBR, or HA-AT1AR were treated with MDC (400 μM) for 30 min before stimulation with isoproterenol (10 μM), endothelin-1 (0.1 μM), or angiotensin II (1 μM), respectively. Data are mean ± SEM of four experiments done in duplicate. **, P < 0.01.
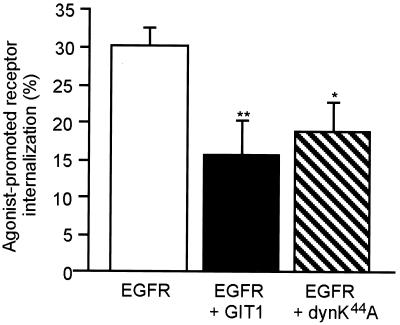
Finally, to distinguish whether GIT1 plays a specific role in agonist-dependent G protein-coupled receptor internalization through the clathrin-coated pit pathway or more generally in clathrin-mediated endocytosis, we examined the effect of GIT1 overexpression on two non-G protein-coupled receptors known to internalize by means of the clathrin pits: the EGF receptor, a tyrosine kinase receptor, and the transferrin receptor, a constitutively recycling receptor (45). Signaling receptors, such as receptor tyrosine kinases and G protein-coupled receptors, require ligand binding for internalization. Similar to most G protein-coupled receptors, phosphorylation of the EGF receptor is necessary for internalization (10, 46, 47). Furthermore, agonist-promoted internalization of G protein-coupled receptors and tyrosine kinase receptors is associated with the recruitment of clathrin to the plasma membrane (12, 48–50). First, we examined the effect of GIT1 on EGF receptor internalization. Overexpression of GIT1, as well as dynamin K44A, significantly reduced agonist-promoted sequestration of the EGF receptor from the cell surface (Fig. 4). These results demonstrate that the effect of GIT1 on agonist-promoted sequestration of receptors is not limited to G protein-coupled receptors.
Figure 4.
Effect of GIT1 overexpression on agonist-promoted internalization of the EGF receptor. HEK 293 cells and GIT1 stably overexpressing cells were transiently transfected with the EGF receptor. Wild-type cells overexpressing the EGF receptor were also transiently transfected with dyn K44A. Cells were stimulated with EGF for 20 min, and EGF receptor internalization was assayed as described in Materials and Methods. Values represent the mean ± SEM from five independent experiments done in duplicate. *, P < 0.01; **, P < 0.001.
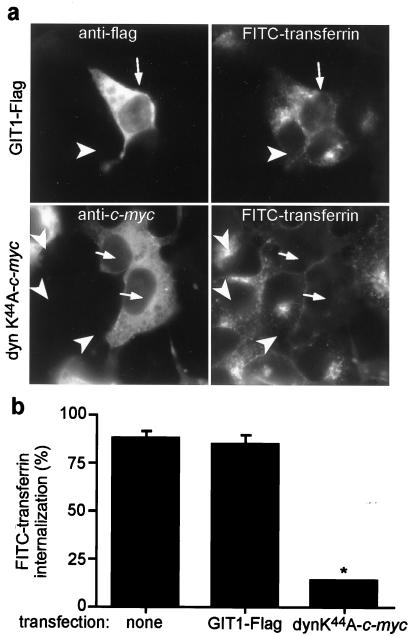
Secondly, we examined whether GIT1 overexpression affected transferrin uptake through the transferrin receptor. This receptor is continually removed from the cell surface through internalization in clathrin-coated vesicles. However, in contrast to G protein-coupled receptors and the EGF receptor, recruitment of this receptor to the pits and its subsequent internalization does not require occupancy of the receptor by transferrin. Immunofluorescence microscopy was used to assess changes in the transferrin accumulation by measuring FITC staining in cells overexpressing GIT1 or dynamin II K44A. Fig. 5a shows a representative field of COS 7 cells overexpressing GIT1-Flag (Upper) or dynamin II K44A-c-myc (Lower) identified by immunostaining (Left). The arrows point to cells overexpressing GIT1-Flag (Upper) and dynamin II K44A (Lower), whereas the arrowheads point to the nontransfected cells. These cells were incubated in the presence of FITC-transferrin for 15 min. The right panels show FITC-transferrin staining of the same fields presented in the left panels. As illustrated in the Upper Left, the GIT1-Flag overexpressing cell (arrow) shows the same FITC-transferrin internalization (Right) as adjacent cells not overexpressing GIT1 (arrowhead). The transferrin appears in both cell types as speckling within endosomal compartments of the cytosol. In contrast, dynamin II K44A-overexpressing cells (Lower Left; arrows) show a marked decrease in FITC-transferrin uptake seen as a decrease in cytosolic localization (Lower Right; arrows). These results are quantitatively represented in Fig. 5b, where GIT1-Flag overexpression does not affect FITC-transferrin uptake, whereas dynamin-dominant negative protein overexpression markedly inhibits uptake. These results show that constitutive internalization through clathrin-coated pits is not affected by GIT1. Thus, the locus of GIT1 action appears to be consistent with an inhibition of agonist-activated receptor recruitment to or internalization by means of the clathrin-coated pits, rather than an inhibition of clathrin-coated pit formation and/or internalization in general.
Figure 5.
Effect of GIT1 overexpression on transferrin uptake. (a) Immunofluorescence of COS 7 cells that have been incubated with FITC-transferrin for 15 min. (Left) A representative field of cells transiently transfected with either GIT1-Flag (Upper) or dyn K44A-c-myc (Lower), identified by staining with anti-Flag and anti-c-myc antibodies, respectively (Left). (Right) The fluorescence signal of cells from the same field shown on the left that have internalized FITC-transferrin. The arrows point to cells overexpressing GIT1-Flag (Upper) and dynamin II K44A (Lower), whereas the arrowheads point to the nontransfected cells. (b) Quantitative assessment of internalized FITC-transferrin. FITC-transferrin uptake was quantified by assessing the number of transfected cells (GIT1-Flag or dynamin II K44A) that have internalized FITC-transferrin. Results are expressed as percent of FITC-transferrin internalization. Data are means ± SEM of three experiments in which 25–40 cells have been evaluated. *, P < 0.001
Discussion
In the present work, we demonstrate that GIT1 is involved in signaling and trafficking of G protein-coupled receptors that are internalized by means of the clathrin-coated pit pathway, but not in the regulation of receptors that internalize by means of other mechanisms. Considerable efforts have been made to define the sequence of events initiating and leading to the agonist-stimulated sequestration of G protein-coupled receptors. The use of dominant negative mutant proteins and biochemical inhibitors has been very helpful in delineating the role of numerous proteins in endocytic pathways. For example, recent studies have revealed an involvement of the β-arrestin and dynamin proteins for at least some receptors.
In this study, we used two inhibitory mutants of β-arrestin and one of dynamin to elucidate the internalization pathways used by several receptor types, some of which are sensitive to GIT1 and others that are not. We have noted that receptors that sequester by means of a β-arrestin-sensitive pathway are also sensitive to GIT1, whereas receptors that sequester by means of a β-arrestin-insensitive pathway are not sensitive to GIT1. Analysis of a number of receptor subtypes has allowed us to define three patterns of sequestration inhibitor sensitivity: (I) internalization through a GIT1-, β-arrestin-, and dynamin-sensitive pathway (β2AR, β1AR, μOR, M1 MR), (II) internalization through a dynamin-sensitive pathway that is insensitive to GIT1 or β-arrestin (VIP1R, ETBR), and (III) internalization through a pathway that is insensitive to GIT1, β-arrestin, or dynamin (AT1AR, M2 MR) (Table 1).
According to the classification recently proposed by Bunemann et al. (51), the GIT1-sensitive pathway (pathway I) corresponds to the clathrin-coated pit pathway. Our results suggest that the internalization mechanism sensitive to GIT1 is indeed the clathrin pathway because the biochemical inhibitor MDC also blocks endocytosis of the β2AR, a representative of the class of receptor that is internalized by means of pathway I, whereas internalization of the ETBR (pathway II) and AT1AR (pathway III) is not significantly affected. We conclude that the distinct patterns of inhibitor sensitivity that we have observed likely correspond to physically distinct cellular processes, although which precise vesicle coats are involved in the nonclathrin-mediated processes remains to be defined for each receptor type. We speculate that pathway II is the caveolae pathway because internalization of receptors in that class is sensitive to a mutant of dynamin. Dynamin has been shown to localize to caveolae structures (23, 24), providing further evidence that this protein may be involved in caveolae-mediated endocytosis. Interestingly, the endothelin receptors have been shown to localize to caveolae (52, 53) in different cell types. Thus, it is possible that they use this pathway of internalization in a cell-specific manner. The dynamin-independent pathway used by the AT1AR and M2 MR (pathway III) may require the participation of yet another type of internalization machinery, the components of which remain unidentified. However, Zhang et al. (38) have shown that the AT1AR internalization can be enhanced by overexpression of β-arrestin 1, suggesting plasticity in the choice of endocytic pathways used by a receptor.
Interestingly, the role of GIT1 in endocytosis does not appear to be limited to G protein-coupled receptors but does appear to be specific for agonist-promoted clathrin-mediated internalization. Indeed, overexpression of GIT1 affects the sequestration of a tyrosine kinase receptor (EGF receptor), but not that of a constitutively recycling receptor (transferrin receptor), both of which internalize by means of the clathrin-coated pit pathway. The major difference between agonist-promoted and constitutive endocytosis is that the adaptor recognition signal is constitutively accessible in the latter but cryptic in the former, until agonist binding has occurred. This model is attractive for the G protein-coupled receptors because GIT1 binds the GRKs that phosphorylate only activated G protein-coupled receptors (7). However, neither GIT1 nor GRKs are known to have any association with the EGF receptor. The link between activated signaling receptors (G protein-coupled receptors and tyrosine kinase receptors) and GIT1 provided by the GRKs or other adaptor proteins may be important for receptor recruitment to the clathrin-coated pits. Our results suggest that GIT1 may play a role in targeting activated receptors to clathrin-coated pits rather than in pit formation or internalization per se. However, further experimentation needs to be done to address this point.
Further, we previously have shown that the ARF GAP domain of GIT1 is critical for its ability to reduce endocytosis and signaling of the β2AR (7), implying that ARF proteins are important for regulating clathrin-mediated endocytosis of G protein-coupled receptors. Moreover, a role for ARF6 in the internalization of the transferrin receptor has been shown previously (54). It therefore was surprising that GIT1 did not affect transferrin receptor function. Perhaps, despite overexpression, GIT1 may require interaction with other signaling proteins for recruitment to the activated ARF6 pool on the plasma membrane, and is thus unable to alter transferrin receptor cycling. Moreover, Cao et al. (55) have suggested that distinct subpopulations of clathrin-coated pits are responsible for endocytosis of the β2AR and the transferrin receptor. Ligand-induced endocytosis is likely to involve components different from those engaged by a constitutively cycling receptor.
In this study, we have defined the specificity of the GIT1 protein for regulating cell surface receptor internalization. Whether the effect of GIT1 solely depends on its ARF GAP activity remains to be defined. However, the study of ARF regulatory proteins like GIT1 will give us more insight into the precise role of these proteins in regulating well-defined internalization pathways such as the clathrin-coated pit endocytic pathway. GIT1 also provides a useful tool to distinguish the different routes of internalization used by G protein-coupled receptors.
Acknowledgments
We thank Drs. Tim Palmer for human A2B receptor cDNA, Gordan Gill for human EGF receptor cDNA, and Marc G. Caron for μOR cDNA. We thank Donna Addison and Mary Holben for secretarial assistance. J.K.L.W. is the recipient of a postdoctoral fellowship from the Medical Research Council of Canada. This work was supported by National Institutes of Health Grant HL 16037 (to R.J.L.). R.J.L. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- GRK
G protein-coupled receptor kinase
- ARF
ADP ribosylation factor
- GAP
GTPase activating protein
- β2AR
β2-adrenergic receptor
- β1AR
β1-adrenergic receptor
- A2BR
adenosine 2B receptor
- VIP1R
vasoactive intestinal peptide 1 receptor
- ETBR
endothelin B receptor
- μOR
μ-opioid receptor
- MR
muscarinic receptor
- AT1AR
angiotensin 1A receptor
- EGFR
epidermal growth factor receptor
- HA
hemagglutinin
- MDC
monodansylcadaverine
References
- 1.Casey P J, Gilman A G. J Biol Chem. 1988;263:2577–2580. [PubMed] [Google Scholar]
- 2.Lefkowitz R J. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher J A, Freedman N J, Lefkowitz R J. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson S S, Downey W E, III, Colapietro A M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 5.Sibley D R, Strasser R H, Benovic J L, Daniel K, Lefkowitz R J. Proc Natl Acad Sci USA. 1986;83:9408–9412. doi: 10.1073/pnas.83.24.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu S S, Lefkowitz R J, Hausdorff W P. J Biol Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- 7.Premont R T, Claing A, Vitale N, Freeman J L R, Pitcher J A, Patton W A, Moss J, Vaughan M, Lefkowitz R J. Proc Natl Acad Sci USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner C E, Brown M C, Perrotta J A, Riedy M C, Nikolopoulos S N, McDonald A R, Bagrodia S, Thomas S, Leventhal P S. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagrodia S, Bailey D, Lenard Z, Hart M, Guan J L, Premont R T, Taylor S J, Cerione R A. J Biol Chem. 1999;274:22393–22400. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- 10.Lamaze C, Schmid S L. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 11.Schmid S L. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 12.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 13.Goodman O B, Jr, Krupnick J G, Gurevich V V, Benovic J L, Keen J H. J Biol Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 14.Krupnick J G, Goodman O B, Jr, Keen J H, Benovic J L. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 15.Laporte S A, Oakley R H, Zhang J, Holt J A, Ferguson S S, Caron M G, Barak L S. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald P H, Cote N L, Lin F T, Premont R T, Pitcher J A, Lefkowitz R J. J Biol Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 17.Mellman I. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 18.Takel K, McPherson P S, Schmid S L, De Camilli P. Nature (London) 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 19.Hinshaw J E, Schmid S L. Nature (London) 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 20.Shpetner H S, Herskovits J S, Vallee R B. J Biol Chem. 1996;271:13–16. doi: 10.1074/jbc.271.1.13. [DOI] [PubMed] [Google Scholar]
- 21.Wang L H, Sudhof T C, Anderson R G. J Biol Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- 22.van der Bliek A M, Redelmeier T E, Damke H, Tisdale E J, Meyerowitz E M, Schmid S L. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henley J R, Krueger E W, Oswald B J, McNiven M A. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh P, McIntosh D P, Schnitzer J E. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- 26.von Zastrow M, Kobilka B K. J Biol Chem. 1994;269:18448–18452. [PubMed] [Google Scholar]
- 27.Tang Y, Hu L A, Miller W E, Ringstad N, Hall R A, Pitcher J A, DeCamilli P, Lefkowitz R J. Proc Natl Acad Sci USA. 1999;96:12559–12564. doi: 10.1073/pnas.96.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Ferguson S S, Barak L S, Bodduluri S R, Laporte S A, Law P Y, Caron M G. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman N J, Ament A S, Oppermann M, Stoffel R H, Exum S T, Lefkowitz R J. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- 30.Oppermann M, Freedman N J, Alexander R W, Lefkowitz R J. J Biol Chem. 1996;271:13266–13272. doi: 10.1074/jbc.271.22.13266. [DOI] [PubMed] [Google Scholar]
- 31.Cullen B R. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 32.Barak L S, Tiberi M, Freedman N J, Kwatra M M, Lefkowitz R J, Caron M G. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 33.Achiriloaie M, Barylko B, Albanesi J P. Mol Cell Biol. 1999;19:1410–1415. doi: 10.1128/mcb.19.2.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Barak L S, Winkler K E, Caron M G, Ferguson S S. J Biol Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 35.Lin F T, Krueger K M, Kendall H E, Daaka Y, Fredericks Z L, Pitcher J A, Lefkowitz R J. J Biol Chem. 1997;272:31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 36.Damke H, Baba T, Warnock D E, Schmid S L. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosaka T, Ikeda K. J Cell Biol. 1983;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Ferguson S S G, Barak L S, Menard L, Caron M G. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 39.Vogler O, Bogatkewitsch G S, Wriske C, Krummenerl P, Jakobs K H, van Koppen C J. J Biol Chem. 1998;273:12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- 40.Vogler O, Nolte B, Voss M, Schmidt M, Jakobs K H, van Koppen C J. J Biol Chem. 1999;274:12333–12338. doi: 10.1074/jbc.274.18.12333. [DOI] [PubMed] [Google Scholar]
- 41.Whistler J L, von Zastrow M. Proc Natl Acad Sci USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pals-Rylaarsdam R, Gurevich V V, Lee K B, Ptasienski J A, Benovic J L, Hosey M M. J Biol Chem. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]
- 43.von Zastrow M, Kobilka B K. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 44.Phonphok Y, Rosenthal K S. FEBS Lett. 1991;281:188–190. doi: 10.1016/0014-5793(91)80390-o. [DOI] [PubMed] [Google Scholar]
- 45.Pearse B M, Robinson M S. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson S S, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- 47.Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–351. [PubMed] [Google Scholar]
- 48.Connolly J L, Green S A, Greene L A. J Cell Biol. 1984;98:457–465. doi: 10.1083/jcb.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corvera S. J Biol Chem. 1990;265:2413–2416. [PubMed] [Google Scholar]
- 50.Grimes M L, Zhou J, Beattie E C, Yuen E C, Hall D E, Valletta J S, Topp K S, LaVail J H, Bunnett N W, Mobley W C. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bunemann M, Lee K B, Pals-Rylaarsdam R, Roseberry A G, Hosey M M. Annu Rev Physiol. 1999;61:169–192. doi: 10.1146/annurev.physiol.61.1.169. [DOI] [PubMed] [Google Scholar]
- 52.Chun M, Liyanage U K, Lisanti M P, Lodish H F. Proc Natl Acad Sci USA. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teixeira A, Chaverot N, Schroder C, Strosberg A D, Couraud P O, Cazaubon S. J Neurochem. 1999;72:120–128. doi: 10.1046/j.1471-4159.1999.0720120.x. [DOI] [PubMed] [Google Scholar]
- 54.D'Souza-Schorey C, Li G, Colombo M I, Stahl P D. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 55.Cao T T, Mays R W, von Zastrow M. J Biol Chem. 1998;273:24592–24602. doi: 10.1074/jbc.273.38.24592. [DOI] [PubMed] [Google Scholar]