Abstract
The ability of synthetic peptides encompassing almost the entire sequence of snRNP U1A polypeptide to be recognized in ELISA by sera of autoimmune patients was investigated. Sera from 18 patients with mixed connective tissue disease (MCTD), 145 with systemic lupus erythematosus (SLE) and 120 with other rheumatic autoimmune diseases were tested with 13 overlapping peptides. Among them, peptide 257-282 and, to a lower extent, peptide 1-11 were recognized by MCTD, SLE and Sjögren's syndrome sera. In contrast, peptide 35-58 was recognized by 94% of MCTD and only 19% of SLE sera. It did not react with any of the other patient sera. The ELISA results were compared with the pattern of reactivity observed in immunoblotting. The results indicate that peptide 35-58 probably contains a major epitope recognized by MCTD autoantibodies. It is noteworthy that in snRNP particles, this region of U1A interacts with RNA and presents only limited homology with the corresponding sequence 32-50 of U2B''.
Full text
PDF



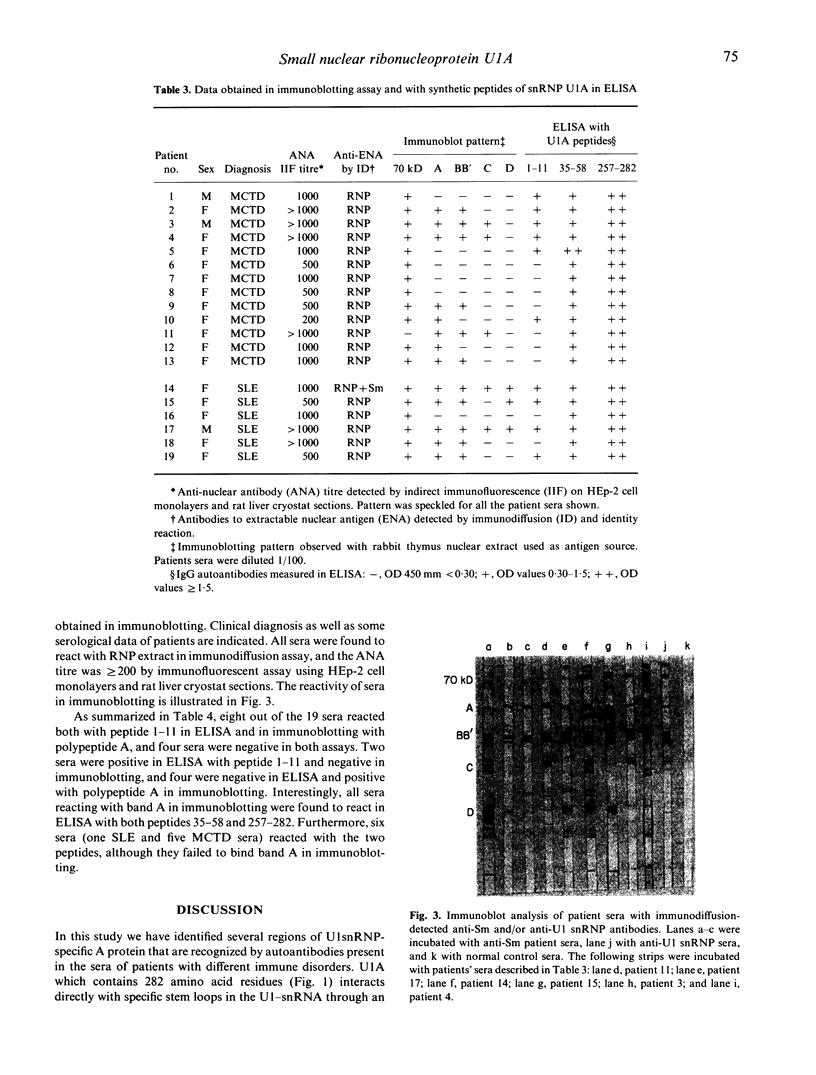



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuaf N., Johanet C., Chretien P., Absalon B. I., Homberg J. C., Buri J. F. Detection of autoantibodies to Sm antigen in systemic lupus erythematosus by immunodiffusion, ELISA and immunoblotting: variability of incidence related to assays and ethnic origin of patients. Eur J Clin Invest. 1990 Aug;20(4):354–359. doi: 10.1111/j.1365-2362.1990.tb01870.x. [DOI] [PubMed] [Google Scholar]
- Abuaf N., Prost A. C., Rouquette-Gally A. M., Bedda N., Fermanian J., Homberg J. C. Comparaison cellules tumorales humaines (Hep 2) et foie de rat pour la détection des anticorps antinucléaires en immunofluorescence indirecte. Ann Biol Clin (Paris) 1984;42(5):363–369. [PubMed] [Google Scholar]
- Barakat S., Briand J. P., Weber J. C., van Regenmortel M. H., Muller S. Recognition of synthetic peptides of Sm-D autoantigen by lupus sera. Clin Exp Immunol. 1990 Aug;81(2):256–262. doi: 10.1111/j.1365-2249.1990.tb03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O. Isolation of intact Sm/RNP antigens from rabbit thymus. J Immunol. 1983 Jul;131(1):347–351. [PubMed] [Google Scholar]
- Briand J. P., Van Dorsselaer A., Raboy B., Muller S. Total chemical synthesis of ubiquitin using BOP reagent: biochemical and immunochemical properties of the purified synthetic product. Pept Res. 1989 Nov-Dec;2(6):381–388. [PubMed] [Google Scholar]
- Combe B., Rucheton M., Graafland H., Lussiez V., Brunel C., Sany J. Clinical significance of anti-RNP and anti-Sm autoantibodies as determined by immunoblotting and immunoprecipitation in sera from patients with connective tissue diseases. Clin Exp Immunol. 1989 Jan;75(1):18–24. [PMC free article] [PubMed] [Google Scholar]
- Feeney R. J., Sauterer R. A., Feeney J. L., Zieve G. W. Cytoplasmic assembly and nuclear accumulation of mature small nuclear ribonucleoprotein particles. J Biol Chem. 1989 Apr 5;264(10):5776–5783. [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., De Jong B. A., Van der Kemp A., Van Venrooij W. J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989 Oct 15;143(8):2560–2566. [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., Sillekens P. T., De Rooij D. J., Van de Putte L. B., Van Venrooij W. J. Detection of autoantibodies in a quantitative immunoassay using recombinant ribonucleoprotein antigens. Clin Exp Immunol. 1989 May;76(2):172–177. [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., Sillekens P. T., Hoet M. H., McAllister G., Lerner M. R., van Venrooij W. J. Small nuclear RNA-associated proteins are immunologically related as revealed by mapping of autoimmune reactive B-cell epitopes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4674–4678. doi: 10.1073/pnas.86.12.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., Sillekens P. T., Hoet M. H., Schalken J. A., Roebroek A. J., Leunissen J. A., van de Ven W. J., van Venrooij W. J. Analysis of a cDNA clone expressing a human autoimmune antigen: full-length sequence of the U2 small nuclear RNA-associated B" antigen. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2421–2425. doi: 10.1073/pnas.84.8.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W., Hoet M., Bringmann P., Lührmann R., van Venrooij W. Autoantibodies to ribonucleoprotein particles containing U2 small nuclear RNA. EMBO J. 1985 Jun;4(6):1545–1550. doi: 10.1002/j.1460-2075.1985.tb03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P. Protein surface analysis. Methods for identifying antigenic determinants and other interaction sites. J Immunol Methods. 1986 Apr 3;88(1):1–18. doi: 10.1016/0022-1759(86)90045-1. [DOI] [PubMed] [Google Scholar]
- Muller S., Barakat S., Watts R., Joubaud P., Isenberg D. Longitudinal analysis of antibodies to histones, Sm-D peptides and ubiquitin in the serum of patients with systemic lupus erythematosus, rheumatoid arthritis and tuberculosis. Clin Exp Rheumatol. 1990 Sep-Oct;8(5):445–453. [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Pellequer J. L., Westhof E., Van Regenmortel M. H. Predicting location of continuous epitopes in proteins from their primary structures. Methods Enzymol. 1991;203:176–201. doi: 10.1016/0076-6879(91)03010-e. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Wang G., Smith E. I., Wigzell H., Hedfors E., Horn J., Sharp G. C. The use of immunoblotting and immunoprecipitation of (U) small nuclear ribonucleoproteins in the analysis of sera of patients with mixed connective tissue disease and systemic lupus erythematosus. A cross-sectional, longitudinal study. Arthritis Rheum. 1986 Aug;29(8):986–996. doi: 10.1002/art.1780290807. [DOI] [PubMed] [Google Scholar]
- Plaué S., Muller S., van Regenmortel M. H. A branched, synthetic octapeptide of ubiquitinated histone H2A as target of autoantibodies. J Exp Med. 1989 May 1;169(5):1607–1617. doi: 10.1084/jem.169.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard K. M., Cohen M. G. Predicting antigenic determinants of autoantigens. Autoimmunity. 1990;5(4):265–275. doi: 10.3109/08916939009014711. [DOI] [PubMed] [Google Scholar]
- Reuter R., Lührmann R. Immunization of mice with purified U1 small nuclear ribonucleoprotein (RNP) induces a pattern of antibody specificities characteristic of the anti-Sm and anti-RNP autoimmune response of patients with lupus erythematosus, as measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8689–8693. doi: 10.1073/pnas.83.22.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I. M., Cooke A. The role of autoantigen in autoimmunity. Immunol Lett. 1987 Dec;16(3-4):259–263. doi: 10.1016/0165-2478(87)90155-6. [DOI] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Sillekens P. T., Habets W. J., Beijer R. P., van Venrooij W. J. cDNA cloning of the human U1 snRNA-associated A protein: extensive homology between U1 and U2 snRNP-specific proteins. EMBO J. 1987 Dec 1;6(12):3841–3848. doi: 10.1002/j.1460-2075.1987.tb02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeenk R., Westgeest T., Swaak T. Antinuclear antibody determination: the present state of diagnostic and clinical relevance. Scand J Rheumatol Suppl. 1985;56:78–92. doi: 10.3109/03009748509102067. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Strunk K., Surowy C. S., Hoch S. O., Barton D. E., Francke U. The human U1-70K snRNP protein: cDNA cloning, chromosomal localization, expression, alternative splicing and RNA-binding. Nucleic Acids Res. 1987 Dec 23;15(24):10373–10391. doi: 10.1093/nar/15.24.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler D. A., Cavallo T. Anti-RNA polymerase I antibodies: potential role in the induction and progression of murine lupus nephritis. J Immunol. 1987 Apr 1;138(7):2119–2123. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Venrooij W. J., Sillekens P. T. Small nuclear RNA associated proteins: autoantigens in connective tissue diseases. Clin Exp Rheumatol. 1989 Nov-Dec;7(6):635–645. [PubMed] [Google Scholar]
- Woppmann A., Patschinsky T., Bringmann P., Godt F., Lührmann R. Characterisation of human and murine snRNP proteins by two-dimensional gel electrophoresis and phosphopeptide analysis of U1-specific 70K protein variants. Nucleic Acids Res. 1990 Aug 11;18(15):4427–4438. doi: 10.1093/nar/18.15.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H., Willis E. H., Penning C. A., Peebles C. L., Tan E. M., Carson D. A. Human autoantibodies to poly(adenosine diphosphate-ribose) polymerase. J Clin Invest. 1987 Sep;80(3):900–904. doi: 10.1172/JCI113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij D. J., Habets W. J., van de Putte L. B., Hoet M. H., Verbeek A. L., van Venrooij W. J. Use of recombinant RNP peptides 70K and A in an ELISA for measurement of antibodies in mixed connective tissue disease: a longitudinal follow up of 18 patients. Ann Rheum Dis. 1990 Jun;49(6):391–395. doi: 10.1136/ard.49.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]



