Abstract
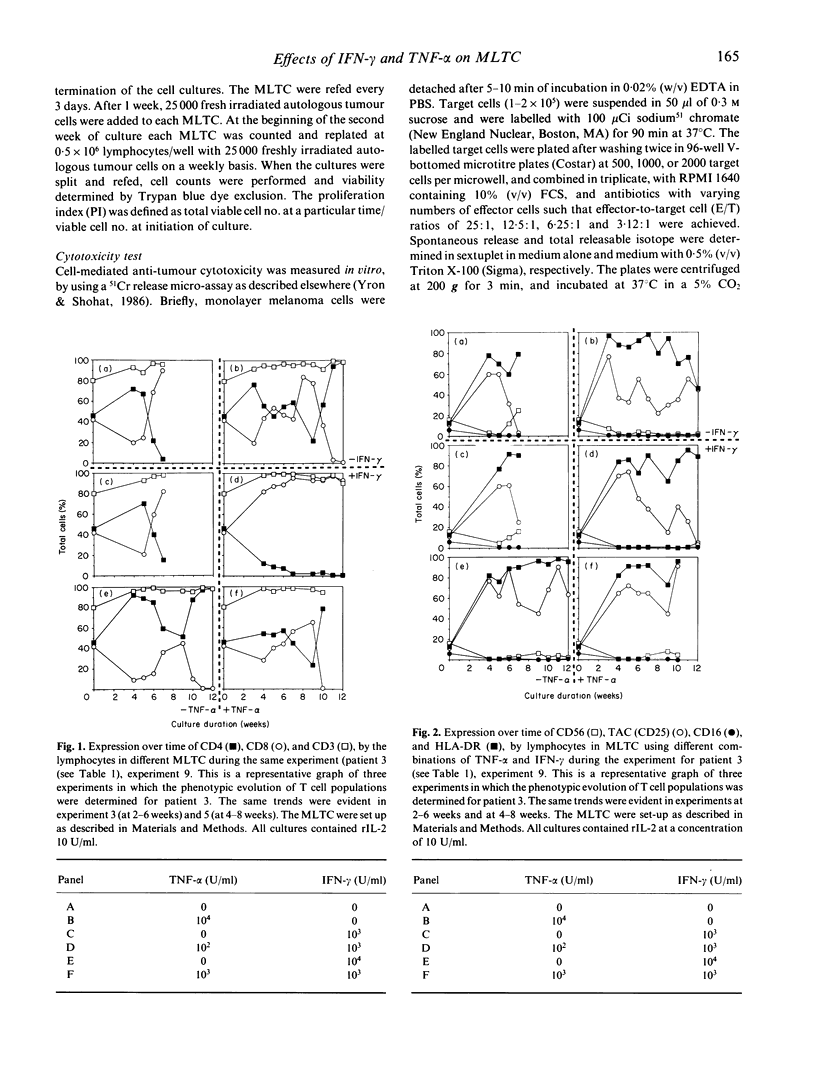
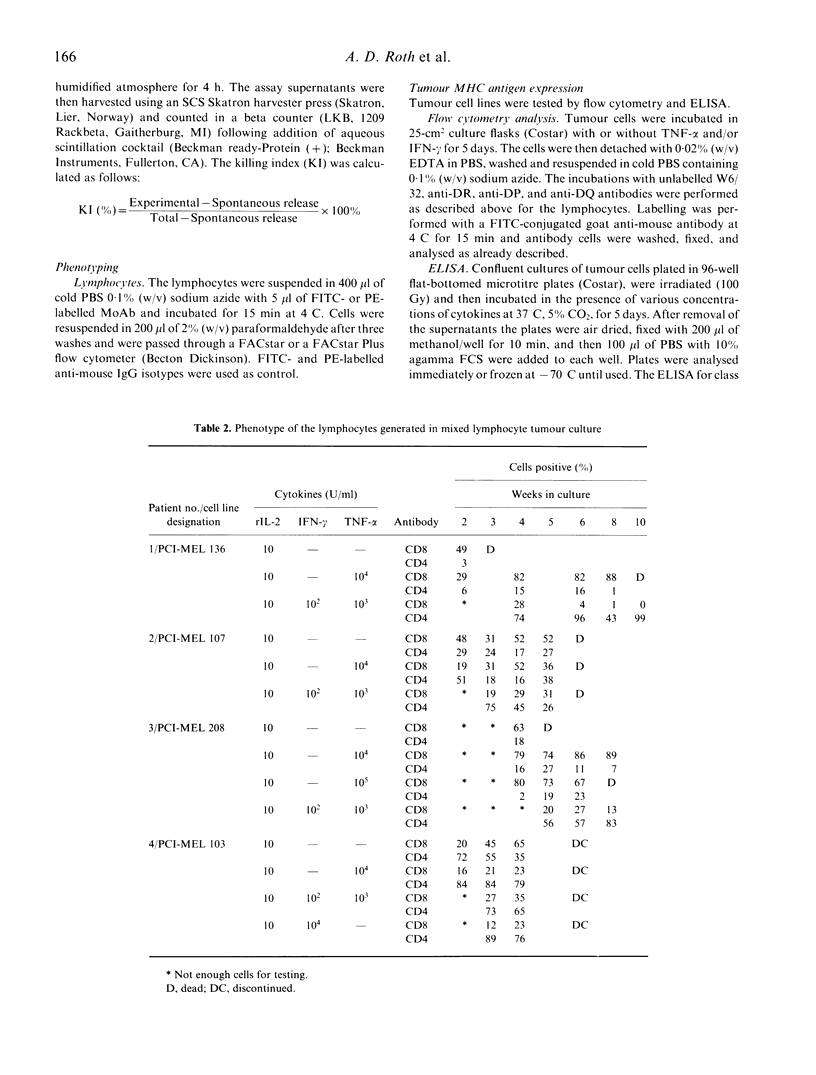
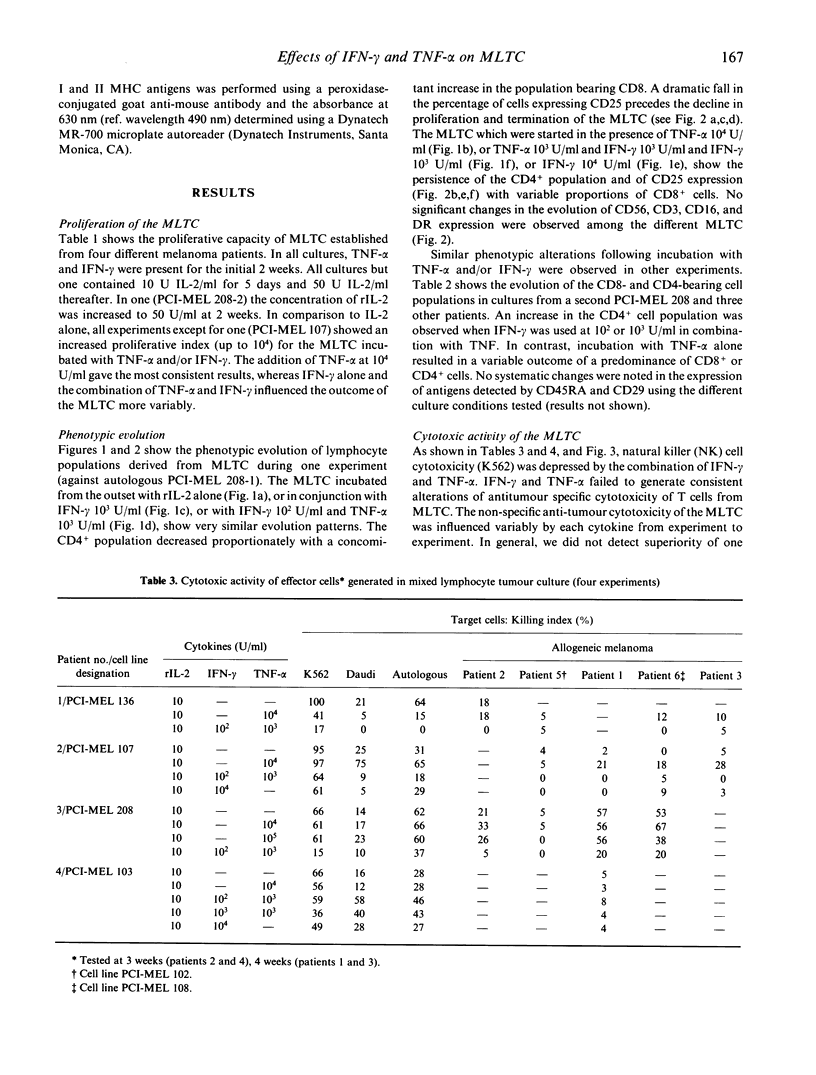
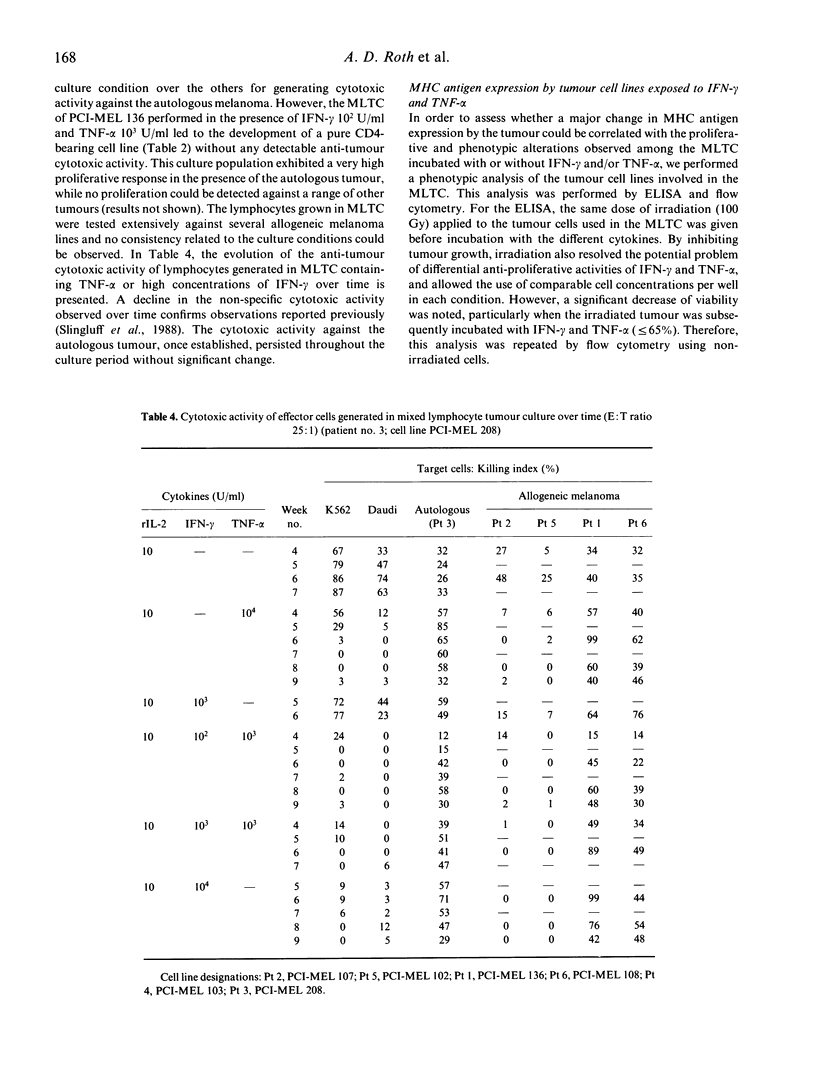
We have studied the influence of tumour necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma) on the development of cytotoxic T lymphocytes (CTL) against melanoma in mixed lymphocyte tumour cultures (MLTC). In these MLTC, TNF-alpha at 10(4) U/ml increased the expansion of the CTL up to 10(4)-fold over recombinant IL-2 (rIL-2) alone. IFN-gamma at 10(4) U/ml and combinations of TNF-alpha plus IFN-gamma at 10(2)-10(3) U/ml promoted the proliferation more variably. MLTC generated with rIL-2 showed a predominance of CD8+ cells, while 2 weeks of culture in the presence of IFN-gamma at 10(4) U/ml, or with IFN-gamma and TNF alpha at 1 x 10(2)-10(3) U/ml, favoured the emergence of CD4+ cell populations. The cytotoxic activity of the lymphocytes generated in these MLTC showed a consistent decline of K562 cytotoxic activity following exposure to the combination of IFN-gamma and TNF-alpha. Despite the altered T cell subset distribution with different combinations of cytokines, no consistent alteration in the specific anti-tumour cytotoxicity against melanoma was detected. These results suggest that TNF-alpha and IFN-gamma influence the activation, phenotypic, and functional outcome of MLTC-generated CTL, and may account for the phenotypic variations observed in T cell populations generated in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belldegrun A., Muul L. M., Rosenberg S. A. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988 Jan 1;48(1):206–214. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Bonnem E. M., Oldham R. K. Gamma-interferon: physiology and speculation on its role in medicine. J Biol Response Mod. 1987 Jun;6(3):275–301. [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Cheever M. A., Thompson J. A., Peace D. J., Greenberg P. D. Potential uses of interleukin 2 in cancer therapy. Immunobiology. 1986 Sep;172(3-5):365–382. doi: 10.1016/S0171-2985(86)80118-8. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Bertoglio J., Blay J. Y., Marchiol-Fournigault C., Fradelizi D. Generation of lymphokine-activated killer cells: synergy between tumor necrosis factor and interleukin 2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6875–6879. doi: 10.1073/pnas.85.18.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Garman R. D., Fan D. P. Characterization of helper factors distinct from interleukin 2 necessary for the generation of allospecific cytolytic T lymphocytes. J Immunol. 1983 Feb;130(2):756–762. [PubMed] [Google Scholar]
- Gillis S., Baker P. E., Ruscetti F. W., Smith K. A. Long-term culture of human antigen-specific cytotoxic T-cell lines. J Exp Med. 1978 Oct 1;148(4):1093–1098. doi: 10.1084/jem.148.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry D., 4th, Alexander M. A., Elder D. E., Herlyn M. F. Interferon-gamma regulates the T cell response to precursor nevi and biologically early melanoma. J Immunol. 1987 Jul 1;139(1):305–312. [PubMed] [Google Scholar]
- Guerry D., 4th, Alexander M. A., Herlyn M. F., Zehngebot L. M., Mitchell K. F., Zmijewski C. M., Lusk E. J. HLA-DR histocompatibility leukocyte antigens permit cultured human melanoma cells from early but not advanced disease to stimulate autologous lymphocytes. J Clin Invest. 1984 Jan;73(1):267–271. doi: 10.1172/JCI111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett R. J., Davis L. S., Lipsky P. E. Comparative effects of tumor necrosis factor-alpha and IL-1 beta on mitogen-induced T cell activation. J Immunol. 1988 Apr 15;140(8):2639–2644. [PubMed] [Google Scholar]
- Holzmann B., Bröcker E. B., Lehmann J. M., Ruiter D. J., Sorg C., Riethmüller G., Johnson J. P. Tumor progression in human malignant melanoma: five stages defined by their antigenic phenotypes. Int J Cancer. 1987 Apr 15;39(4):466–471. doi: 10.1002/ijc.2910390410. [DOI] [PubMed] [Google Scholar]
- Iigo M., Sakurai M., Tamura T., Saijo N., Hoshi A. In vivo antitumor activity of multiple injections of recombinant interleukin 2, alone and in combination with three different types of recombinant interferon, on various syngeneic murine tumors. Cancer Res. 1988 Jan 15;48(2):260–264. [PubMed] [Google Scholar]
- Itoh K., Shiiba K., Shimizu Y., Suzuki R., Kumagai K. Generation of activated killer (AK) cells by recombinant interleukin 2 (rIL 2) in collaboration with interferon-gamma (IFN-gamma). J Immunol. 1985 May;134(5):3124–3129. [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Balch C. M. Interleukin 2 activation of cytotoxic T-lymphocytes infiltrating into human metastatic melanomas. Cancer Res. 1986 Jun;46(6):3011–3017. [PubMed] [Google Scholar]
- Jin B., Scott J. L., Vadas M. A., Burns G. F. TGF beta down-regulates TLiSA1 expression and inhibits the differentiation of precursor lymphocytes into CTL and LAK cells. Immunology. 1989 Apr;66(4):570–576. [PMC free article] [PubMed] [Google Scholar]
- Kern D. E., Gillis S., Okada M., Henney C. S. The role of interleukin-2 (IL-2) in the differentiatin of cytotoxic T cells: the effect of monoclonal anti-IL-2 antibody and absorption with IL-2 dependent T cell lines. J Immunol. 1981 Oct;127(4):1323–1328. [PubMed] [Google Scholar]
- Knuth A., Wölfel T., Klehmann E., Boon T., Meyer zum Büschenfelde K. H. Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2804–2808. doi: 10.1073/pnas.86.8.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Danis K. M., Codias E. K. Tumor necrosis factor synergistically acts with IFN-gamma to regulate Ly-6A/E expression in T lymphocytes, thymocytes and bone marrow cells. J Immunol. 1989 Mar 15;142(6):1929–1936. [PubMed] [Google Scholar]
- Mills B. J., Shively J. E., Mitsky P. S., Hawke D. H., Forman S. J., Wright C. L., Todd C. W. Synergy between interleukin-2 and a second factor in the long-term growth of human T cells. Immunology. 1986 Sep;59(1):57–61. [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Nakano K., Okugawa K., Furuichi H., Matsui Y., Sohmura Y. Augmentation of the generation of cytotoxic T lymphocytes against syngeneic tumor cells by recombinant human tumor necrosis factor. Cell Immunol. 1989 Apr 15;120(1):154–164. doi: 10.1016/0008-8749(89)90183-4. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Gutterman J. U., Grimm E. A. Synergy of tumor necrosis factor and interleukin 2 in the activation of human cytotoxic lymphocytes: effect of tumor necrosis factor alpha and interleukin 2 in the generation of human lymphokine-activated killer cell cytotoxicity. Cancer Res. 1988 Feb 15;48(4):788–792. [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Siegel J. P. Effects of interferon-gamma on the activation of human T lymphocytes. Cell Immunol. 1988 Feb;111(2):461–472. doi: 10.1016/0008-8749(88)90109-8. [DOI] [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Darrow T., Vervaert C., Quinn-Allen M. A., Seigler H. F. Human cytotoxic T cells specific for autologous melanoma cells: successful generation from lymph node cells in seven consecutive cases. J Natl Cancer Inst. 1988 Sep 7;80(13):1016–1026. doi: 10.1093/jnci/80.13.1016. [DOI] [PubMed] [Google Scholar]
- Sone S., Utsugi T., Nii A., Ogura T. Differential effects of recombinant interferons alpha, beta, and gamma on induction of human lymphokine (IL-2)-activated killer activity. J Natl Cancer Inst. 1988 May 18;80(6):425–431. doi: 10.1093/jnci/80.6.425. [DOI] [PubMed] [Google Scholar]
- Takai Y., Herrmann S. H., Greenstein J. L., Spitalny G. L., Burakoff S. J. Requirement for three distinct lymphokines for the induction of cytotoxic T lymphocytes from thymocytes. J Immunol. 1986 Dec 1;137(11):3494–3500. [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Talmadge J. E., Phillips H., Schneider M., Rowe T., Pennington R., Bowersox O., Lenz B. Immunomodulatory properties of recombinant murine and human tumor necrosis factor. Cancer Res. 1988 Feb 1;48(3):544–550. [PubMed] [Google Scholar]
- Taramelli D., Fossati G., Mazzocchi A., Delia D., Ferrone S., Parmiani G. Classes I and II HLA and melanoma-associated antigen expression and modulation on melanoma cells isolated from primary and metastatic lesions. Cancer Res. 1986 Jan;46(1):433–439. [PubMed] [Google Scholar]
- Wang Y. L., Si L. S., Kanbour A., Herberman R. B., Whiteside T. L. Lymphocytes infiltrating human ovarian tumors: synergy between tumor necrosis factor alpha and interleukin 2 in the generation of CD8+ effectors from tumor-infiltrating lymphocytes. Cancer Res. 1989 Nov 1;49(21):5979–5985. [PubMed] [Google Scholar]
- Whiteside T. L., Miescher S., Hurlimann J., Moretta L., von Fliedner V. Clonal analysis and in situ characterization of lymphocytes infiltrating human breast carcinomas. Cancer Immunol Immunother. 1986;23(3):169–178. doi: 10.1007/BF00205646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. L., Wilson D. E., Jenson J. C., Familletti P. C., Stremlo D. L., Gately M. K. Characterization of a factor(s) which synergizes with recombinant interleukin 2 in promoting allogeneic human cytolytic T-lymphocyte responses in vitro. Cell Immunol. 1988 Jan;111(1):39–54. doi: 10.1016/0008-8749(88)90049-4. [DOI] [PubMed] [Google Scholar]
- Yron I., Shohat L. Miniaturization of the standard 51Cr release assay for long term follow-up of NK activity of individual mice. J Immunol Methods. 1986 Nov 6;93(2):193–200. doi: 10.1016/0022-1759(86)90188-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., Gauny S., Chan A., Landre P., Winkelhake J. L. Sequence dependence of administration of human recombinant tumor necrosis factor and interleukin-2 in murine tumor therapy. J Natl Cancer Inst. 1989 Feb 1;81(3):227–231. doi: 10.1093/jnci/81.3.227. [DOI] [PubMed] [Google Scholar]
- Zucali J. R., Elfenbein G. J., Barth K. C., Dinarello C. A. Effects of human interleukin 1 and human tumor necrosis factor on human T lymphocyte colony formation. J Clin Invest. 1987 Sep;80(3):772–777. doi: 10.1172/JCI113133. [DOI] [PMC free article] [PubMed] [Google Scholar]


