Abstract
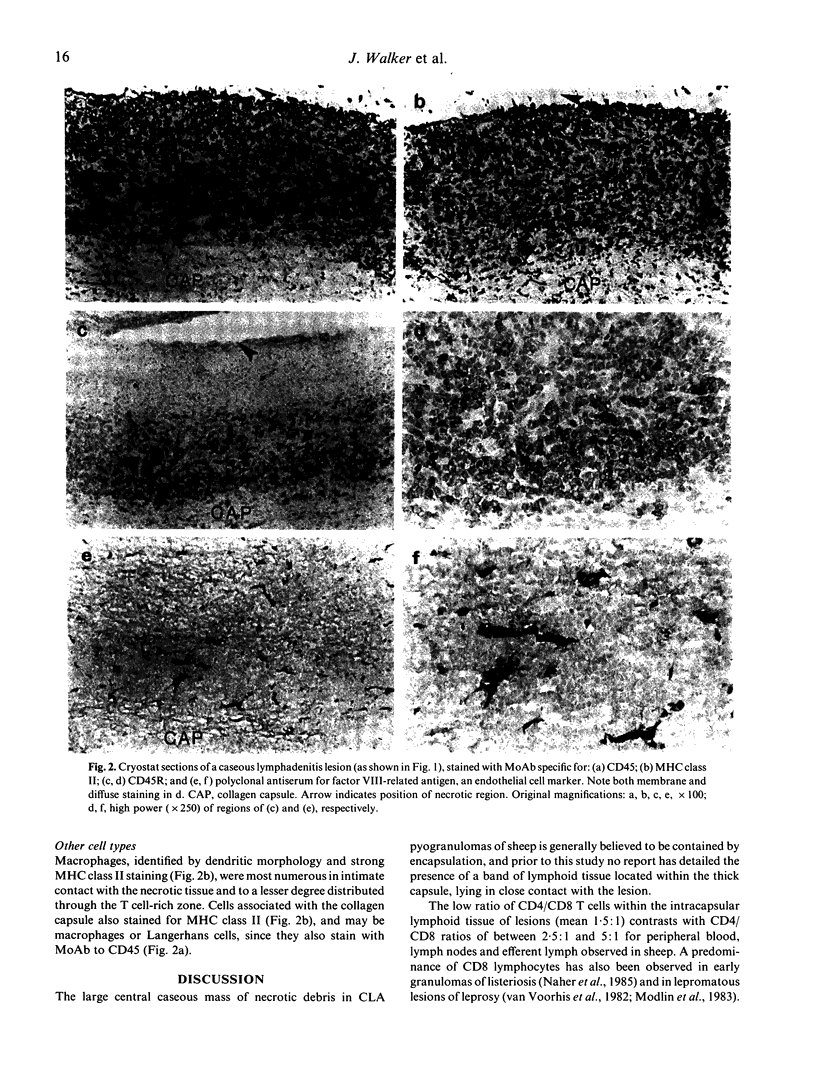
Pyogranulomas of ovine caseous lymphadenitis (CLA) are encapsulated lesions resulting from infections with Corynebacterium pseudotuberculosis, a bacterial pathogen able to grow within macrophages. Immunohistology of CLA lesions showed a band of lymphocytes lining the inside of the collagen capsule in intimate contact with necrotic tissue, the intracapsular lymphocytes being organized into three layers. The innermost layer, immediately adjacent to the central necrotic tissue consisted of a narrow band of MHC class II staining macrophages. Cells staining for CD4, CD8 and gamma delta T cell markers were unevenly distributed throughout the lymphoid layer, tending to be more numerous immediately external to the macrophage layer. The intracapsular lymphoid tissue contained a high proportion of CD8+ lymphocytes (CD4:CD8, 1.5:1) and of gamma delta lymphocytes (CD4:CD8:gamma delta, 1:0.7:0.8). External to the T cell-rich zone and adjacent to the surrounding collagen capsule was a dense band of cells, a proportion of which stained atypically for CD45R and were tentatively identified as B cells. CD8+ and gamma delta+ T cells showed similar distributions and their relative abundance, compared with CD4+ T cells, was a distinguishing feature of the CLA lesion. Staining for factor VIII-related antigen clearly showed endothelial venules throughout the intracapsular lymphoid tissue. The presence of endothelial venules and the organized architecture of the lymphoid tissue teleologically argues that lymphocytes are continually recruited into chronic CLA lesions and play an important role in the ongoing disease process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batey R. G. Frequency and consequence of caseous lymphadenitis in sheep and lambs slaughtered at a Western Australian abattoir. Am J Vet Res. 1986 Feb;47(2):482–485. [PubMed] [Google Scholar]
- Batey R. G. Pathogenesis of caseous lymphadenitis in sheep and goats. Aust Vet J. 1986 Sep;63(9):269–272. doi: 10.1111/j.1751-0813.1986.tb08064.x. [DOI] [PubMed] [Google Scholar]
- Burrell D. H. Experimental induction of caseous lymphadenitis in sheep by intralymphatic inoculation of Corynebacterium ovis. Res Vet Sci. 1978 May;24(3):269–276. [PubMed] [Google Scholar]
- Hard G. C. Comparative toxic effect of the surface lipid of Corynebacterium ovis on peritoneal macrophages. Infect Immun. 1975 Dec;12(6):1439–1449. doi: 10.1128/iai.12.6.1439-1449.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard G. C. Examination by electron microscopy of the interaction between peritoneal phagocytes and Corynebacterium ovis. J Med Microbiol. 1972 Nov;5(4):483–491. doi: 10.1099/00222615-5-4-483. [DOI] [PubMed] [Google Scholar]
- Jolly R. D. The pathogenic action of the exotoxin of Corynebacterium ovis. J Comp Pathol. 1965 Oct;75(4):417–431. doi: 10.1016/0021-9975(65)90022-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Hein W. R. A large proportion of bovine T cells express the gamma delta T cell receptor and show a distinct tissue distribution and surface phenotype. Int Immunol. 1989;1(5):540–545. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Brandon M. R. A monoclonal antibody to the p220 component of sheep LCA identifies B cells and a unique lymphocyte subset. Cell Immunol. 1987 Nov;110(1):46–55. doi: 10.1016/0008-8749(87)90100-6. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Meeusen E., Gorrell M. D., Rickard M. D., Brandon M. R. Lymphocyte subpopulations of sheep in protective immunity to Taenia hydatigena. Parasite Immunol. 1989 Mar;11(2):169–181. doi: 10.1111/j.1365-3024.1989.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Gebhard J. F., Taylor C. R., Rea T. H. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol. 1983 Jul;53(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Melancon-Kaplan J., Young S. M., Pirmez C., Kino H., Convit J., Rea T. H., Bloom B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Muckle C. A., Gyles C. L. Relation of lipid content and exotoxin production to virulence of Corynebacterium pseudotuberculosis in mice. Am J Vet Res. 1983 Jun;44(6):1149–1153. [PubMed] [Google Scholar]
- Näher H., Sperling U., Takacs L., Hahn H. Dynamics of T cells of L3T4 and Ly 2 phenotype within granulomas in murine listeriosis. Clin Exp Immunol. 1985 Jun;60(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- Onon E. O. Purification and partial characterization of the exotoxin of Corynebacterium ovis. Biochem J. 1979 Jan 1;177(1):181–186. doi: 10.1042/bj1770181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehested M., Hou-Jensen K. Factor VII related antigen as an endothelial cell marker in benign and malignant diseases. Virchows Arch A Pathol Anat Histol. 1981;391(2):217–225. doi: 10.1007/BF00437598. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]




