Abstract
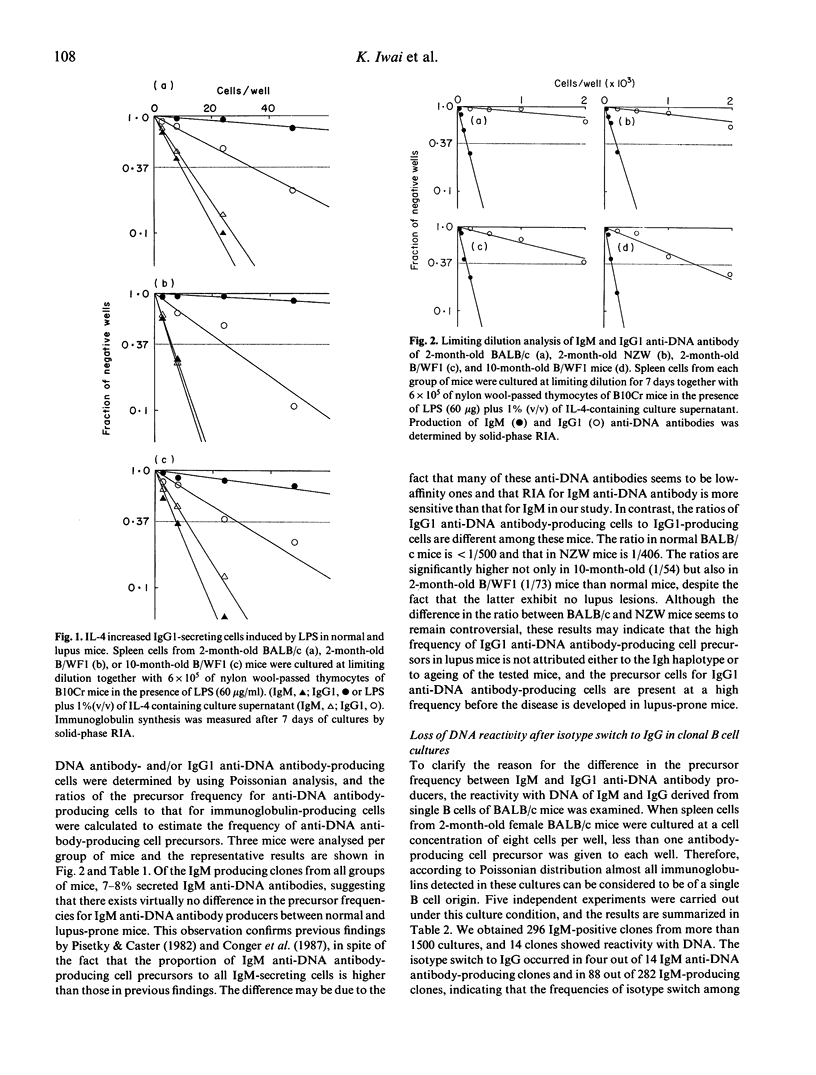
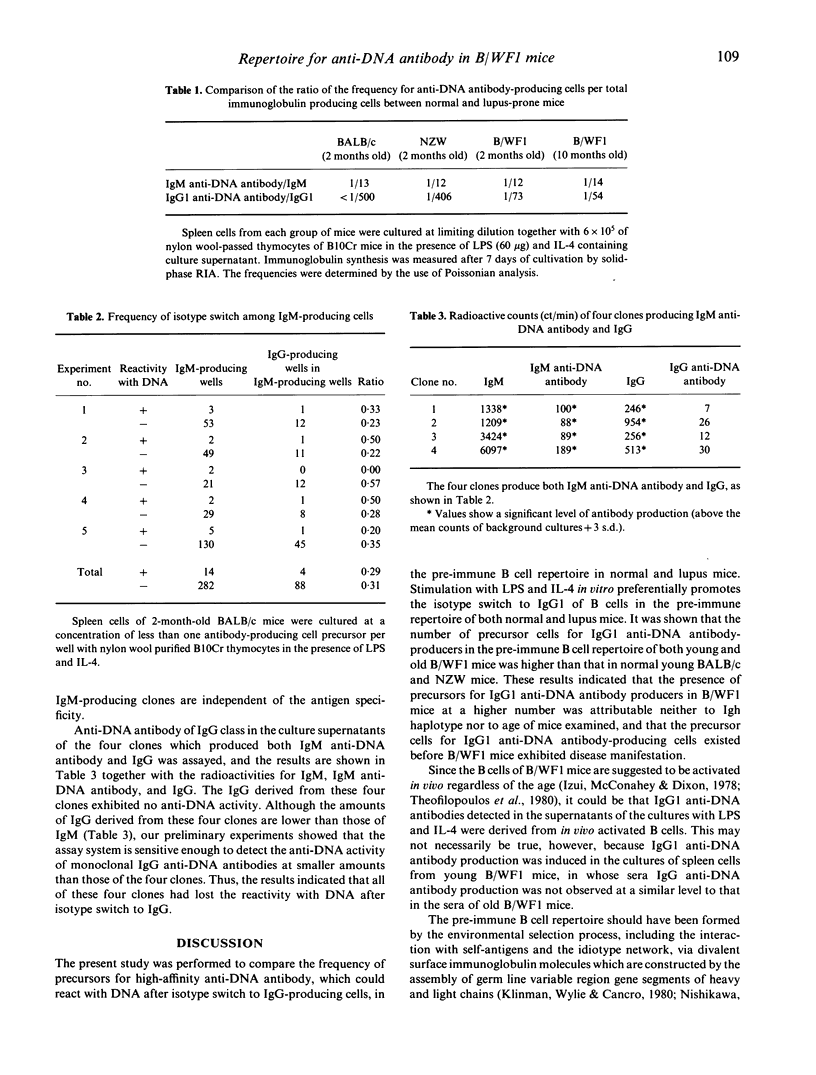
The precursor frequency for anti-DNA antibody-producing cells in the pre-immune B cell repertoire was investigated in young female BALB/c and NZW mice, and in young and aged female NZB x NZWF1 (B/WF1) mice. Spleen cells from these mice were diluted serially and stimulated polyclonally in vitro with lipopolysaccharide (LPS) and IL-4 to induce both IgM and IgG1 production. The results demonstrated that there existed virtually no difference in precursor frequency for IgM anti-DNA antibody-producing cells between normal and lupus mice, confirming previous observations made by other investigators. In contrast, the number of precursors for IgG1 anti-DNA antibody-producing cells was much higher in young and old B/WF1 mice than in normal mice. These results suggest that the high frequency of precursors for IgG1 anti-DNA antibody-producing cells in the pre-immune B cell repertoire of B/WF1 mice is a crucial factor for the pathogenesis of systemic lupus erythematosus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. II. Frequencies of B cells producing antibodies which lyse sheep or horse erythrocytes, and trinitrophenylated or nitroiodophenylated sheep erythrocytes. J Exp Med. 1977 Jun 1;145(6):1520–1530. doi: 10.1084/jem.145.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar S. M., Scharff M. D. Somatic diversification of the S107 (T15) VH11 germ-line gene that encodes the heavy-chain variable region of antibodies to double-stranded DNA in (NZB x NZW)F1 mice. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3970–3974. doi: 10.1073/pnas.85.11.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstedt-Lindqvist S., Moon H. B., Persson U., Möller G., Heusser C., Severinson E. Interleukin 4 instructs uncommitted B lymphocytes to switch to IgG1 and IgE. Eur J Immunol. 1988 Jul;18(7):1073–1077. doi: 10.1002/eji.1830180716. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Pike B. L., Nossal G. J. Clonal analysis of the anti-DNA repertoire of murine B lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2931–2935. doi: 10.1073/pnas.84.9.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdery J. S., Jacobi S. M., Pitts A. K., Tyler T. L. Defective B cell clonal regulation and autoantibody production in New Zealand black mice. J Immunol. 1987 Feb 1;138(3):760–764. [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Eaton R. B., Schnneider G., Schur P. H. Enzyme immunoassay for antibodies to native DNA. Specificity and quality of antibodies. Arthritis Rheum. 1983 Jan;26(1):52–62. doi: 10.1002/art.1780260109. [DOI] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Tron F., Jacob L., Bach J. F. The VH gene sequences of anti-DNA antibodies in two different strains of lupus-prone mice are highly related. Eur J Immunol. 1989 Jul;19(7):1241–1246. doi: 10.1002/eji.1830190714. [DOI] [PubMed] [Google Scholar]
- Eilat D., Webster D. M., Rees A. R. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J Immunol. 1988 Sep 1;141(5):1745–1753. [PubMed] [Google Scholar]
- Holmberg D., Freitas A. A., Portnoï D., Jacquemart F., Avrameas S., Coutinho A. Antibody repertoires of normal BALB/c mice: B lymphocyte populations defined by state of activation. Immunol Rev. 1986 Oct;93:147–169. doi: 10.1111/j.1600-065x.1986.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Izui S., Zaldivar N. M., Scher I., Lambert P. H. Mechanism for induction of anti-DNA antibodies by bacterial lipopolysaccharides in mice. I. Anti-DNA induction by LPS without significant release of DNA in circulating blood. J Immunol. 1977 Dec;119(6):2151–2156. [PubMed] [Google Scholar]
- Jyonouchi H., Kincade P. W., Good R. A., Gershwin M. E. B lymphocyte lineage cells in newborn and very young NZB mice: evidence for regulatory disorders affecting B cell formation. J Immunol. 1983 Nov;131(5):2219–2225. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Eisenberg R. A., Steinberg A. D. Development of the autoimmune B cell repertoire in MRL-lpr/lpr mice. J Immunol. 1990 Jan 15;144(2):506–511. [PubMed] [Google Scholar]
- Klinman N. R. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972 Aug 1;136(2):241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno A., Yoshida H., Sekita K., Maruyama N., Ozaki S., Hirose S., Shirai T. Genetic regulation of the class conversion of dsDNA-specific antibodies in (NZB X NZW)F1 hybrid. Immunogenetics. 1983;18(5):513–524. doi: 10.1007/BF00364392. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J. Clonal analysis of autoantibody-producing cell precursors in the preimmune B cell repertoire. J Immunol. 1988 Dec 15;141(12):4118–4123. [PubMed] [Google Scholar]
- Nishikawa S., Takemori T., Rajewsky K. The expression of a set of antibody variable regions in lipopolysaccharide-reactive B cells at various stages of ontogeny and its control by anti-idiotypic antibody. Eur J Immunol. 1983 Apr;13(4):318–325. doi: 10.1002/eji.1830130409. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Papoian R., Pillarisetty R., Talal N. Immunological regulation of spontaneous antibodies to DNA and RNA. II. Sequential switch from IgM to IgG in NZB/NZW F1 mice. Immunology. 1977 Jan;32(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- Pisetsky D. S., Caster S. A. The B-cell repertoire for autoantibodies: frequency of precursor cells for anti-DNA antibodies. Cell Immunol. 1982 Sep 15;72(2):294–305. doi: 10.1016/0008-8749(82)90477-4. [DOI] [PubMed] [Google Scholar]
- Sawada S., Pillarisetty R. J., Michalski J. P., Palmer D. W., Talal N. Lymphocytes binding polyriboadenylic acid and synthesizing antibodies to nucleic acids in autoimmune and normal mice. J Immunol. 1977 Jul;119(1):355–360. [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. H., Hang L., Barkley J., Fulton R. J., D'Hoostelaere L., Robinson A., Dixon F. J. Isotypes of spontaneous and mitogen-induced autoantibodies in SLE-prone mice. J Immunol. 1984 Mar;132(3):1271–1275. [PubMed] [Google Scholar]
- Steward M. W., Hay F. C. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976 Nov;26(2):363–370. [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Shawler D. L., Eisenberg R. A., Dixon F. J. Splenic immunoglobulin-secreting cells and their regulation in autoimmune mice. J Exp Med. 1980 Feb 1;151(2):446–466. doi: 10.1084/jem.151.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Nishikawa S., Katsura Y., Kumagai S., Imura H. B cell repertoire for anti-DNA antibody in normal and lupus mice: differential expression of precursor cells for high and low affinity anti-DNA antibodies. Clin Exp Immunol. 1988 Jan;71(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Faiferman I., Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977 Jan;59(1):90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Yoshida M., Izui S., Lambert P. H. Distinct clonotypes of anti-DNA antibodies in mice with lupus nephritis. J Clin Invest. 1985 Aug;76(2):685–694. doi: 10.1172/JCI112022. [DOI] [PMC free article] [PubMed] [Google Scholar]


