Abstract
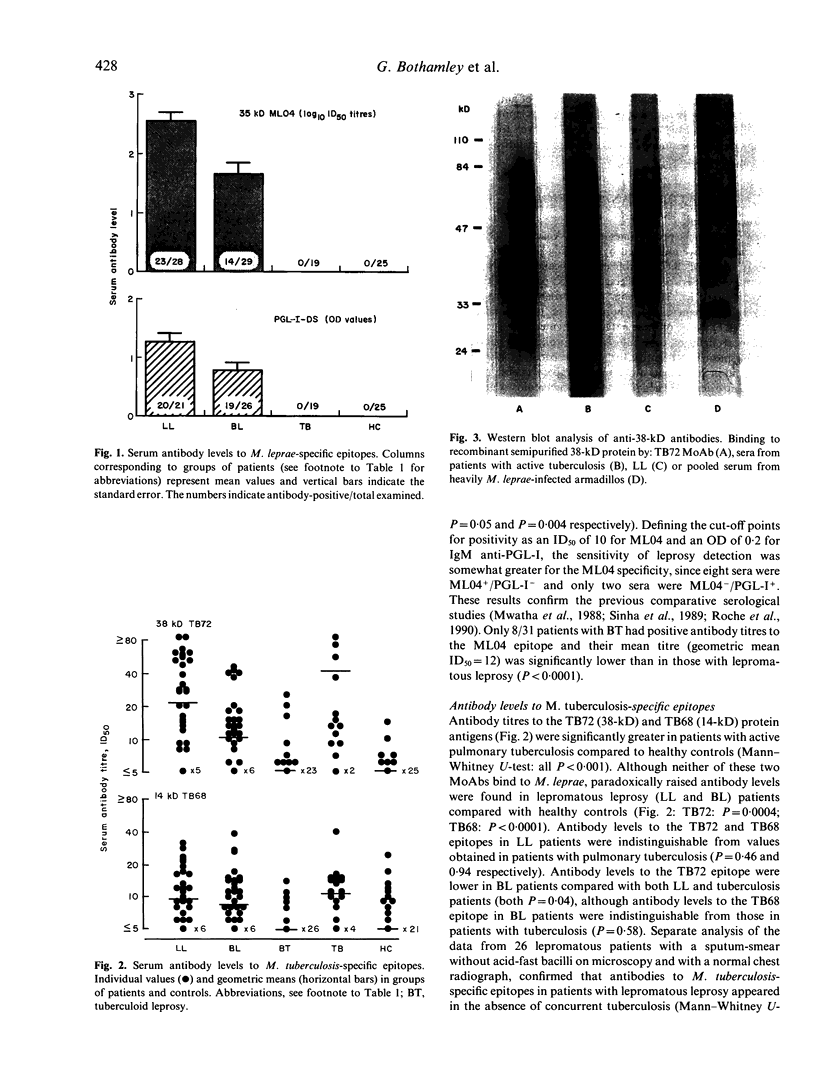
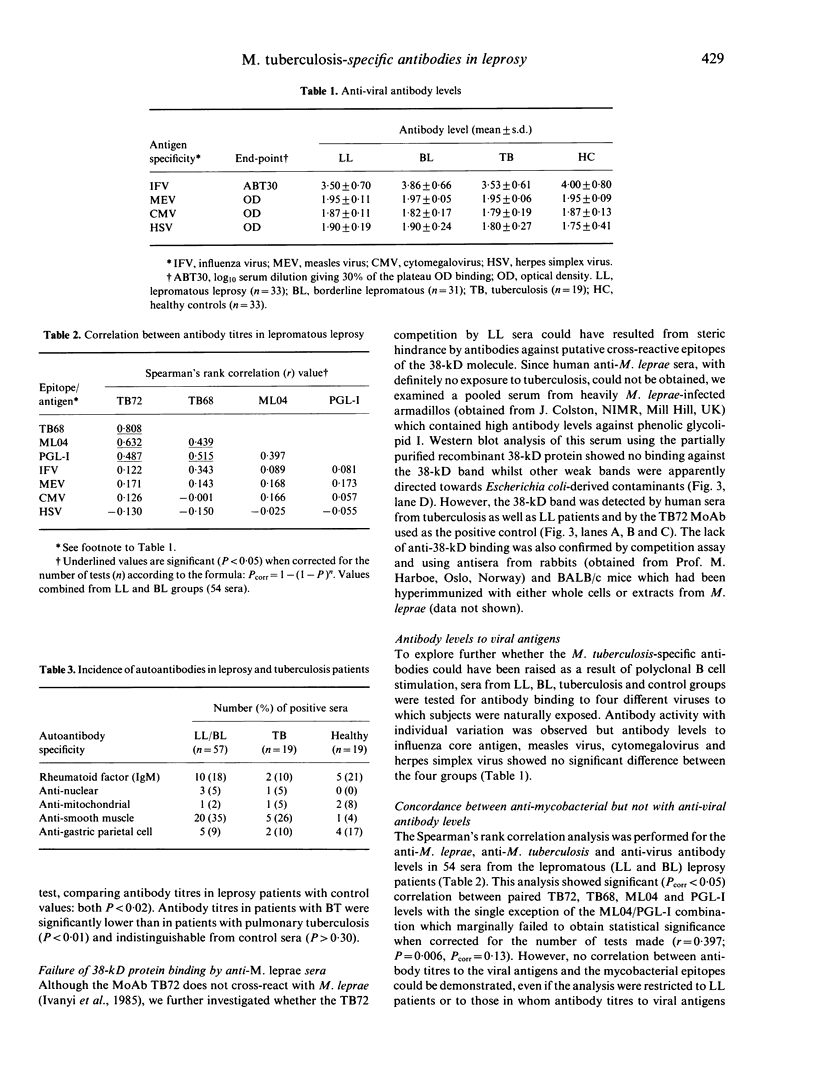
Sera from patients with leprosy or tuberculosis and healthy subjects have been analysed for the presence of antibodies to four species-specific mycobacterial epitopes, four different viruses and five autoantigens. Antibodies to the Mycobacterium leprae-specific 35-kD protein and phenolic glycolipid I epitopes were not present in patients with active pulmonary tuberculosis. In contrast, antibody levels to species-specific epitopes of the 38-kD and 14-kD antigens M. tuberculosis were significantly elevated in patients with lepromatous leprosy. Neither of the two antigens is cross-reactive with M. leprae at the B cell level. However, it was considered that cross-reactive helper T cells could recall the response of M. tuberculosis-specific memory B cells, which had been primed through prior self-healing tuberculous infection. As an alternative explanation, the possible role of polyclonal B cell stimulation was considered. This seemed unlikely, however, since: (i) antibody levels to autoantigens, except anti-smooth muscle, were not elevated, and (ii) antibody levels to four distinct viruses, unlike those to all mycobacterial epitopes, showed no correlation with titres, to M. tuberculosis-specific epitopes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Hansen E. B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989 Aug;57(8):2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G., Udani P., Rudd R., Festenstein F., Ivanyi J. Humoral response to defined epitopes of tubercle bacilli in adult pulmonary and child tuberculosis. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):639–645. doi: 10.1007/BF01964242. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Watson S., Nelson K. E., Schauf V., Makonkawkeyoon S., Jacobson R. R. Aberrant immunoregulatory control of B lymphocyte function in lepromatous leprosy. Clin Exp Immunol. 1982 Jul;49(1):105–114. [PMC free article] [PubMed] [Google Scholar]
- Closs O., Reitan L. J., Negassi K., Harboe M., Belehu A. In vitro stimulation of lymphocytes in leprosy patients, healthy contacts of leprosy patients, and subjects not exposed to leprosy. Comparison of an antigen fraction prepared from Mycobacterium leprae and tuberculin-purified protein derivative. Scand J Immunol. 1982 Aug;16(2):103–115. doi: 10.1111/j.1365-3083.1982.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Cramer M., Braun D. G. Cross-stimulation of monoclonal antibodies in anamnestic responses. J Exp Med. 1973 Dec 1;138(6):1533–1544. doi: 10.1084/jem.138.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., FRANCIS T., Jr Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med. 1953 Dec;98(6):641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch S., Vinit M. A., Bussard A. E. Original antigenic sin at the cellular level. II. Specificity of the antibodies produced by individual cells. Eur J Immunol. 1973 Apr;3(4):235–240. doi: 10.1002/eji.1830030411. [DOI] [PubMed] [Google Scholar]
- East I. J., Todd P. E., Leach S. J. Original antigenic sin: experiments with a defined antigen. Mol Immunol. 1980 Dec;17(12):1539–1544. doi: 10.1016/0161-5890(80)90179-0. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. Leprosy and tuberculosis--an epidemiological comparison. Tubercle. 1984 Jun;65(2):137–153. doi: 10.1016/0041-3879(84)90067-9. [DOI] [PubMed] [Google Scholar]
- Girard G. Quel fut le sort des lépreux au cours de la pandémie pesteuse du moyen age (1348-1350) Bull Soc Pathol Exot Filiales. 1975 Jan-Feb;68(1):33–37. [PubMed] [Google Scholar]
- Halstead S. B., Rojanasuphot S., Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg. 1983 Jan;32(1):154–156. doi: 10.4269/ajtmh.1983.32.154. [DOI] [PubMed] [Google Scholar]
- Harboe M., Ivanyi J. Analysis of monoclonal antibodies to Mycobacterium leprae by crossed immunoelectrophoresis. Scand J Immunol. 1987 Feb;25(2):133–138. doi: 10.1111/j.1365-3083.1987.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Tokuhisa T., Herzenberg L. A. Carrier-priming leads to hapten-specific suppression. Nature. 1980 Jun 26;285(5767):664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Krambovitis E., Keen M. Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin Exp Immunol. 1983 Nov;54(2):337–345. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J. Recall of antibody synthesis to the primary antigen following successive immunization with heterologous albumins. A two-cell theory of the original antigenic sin. Eur J Immunol. 1972 Aug;2(4):354–359. doi: 10.1002/eji.1830020411. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K., Jackett P., Bothamley G. Immunological study of the defined constituents of mycobacteria. Springer Semin Immunopathol. 1988;10(4):279–300. doi: 10.1007/BF02053841. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadival G. V., Chaparas S. D., Hussong D. Characterization of serologic and cell-mediated reactivity of a 38-kDa antigen isolated from Mycobacterium tuberculosis. J Immunol. 1987 Oct 1;139(7):2447–2451. [PubMed] [Google Scholar]
- Kano K., Aranzazu N., Nishimaki T., Convit J., Albini B., Milgrom F. Serological and immunohistological studies on lepromatous leprosy. Int Arch Allergy Appl Immunol. 1981;64(1):19–24. doi: 10.1159/000232670. [DOI] [PubMed] [Google Scholar]
- Kingston A. E., Salgame P. R., Mitchison N. A., Colston M. J. Immunological activity of a 14-kilodalton recombinant protein of Mycobacterium tuberculosis H37Rv. Infect Immun. 1987 Dec;55(12):3149–3154. doi: 10.1128/iai.55.12.3149-3154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist K. J., Coleman R. E., Osterland C. K. Autoantibodies in chronic pulmonary tuberculosis. J Chronic Dis. 1970 Apr;22(11):717–725. doi: 10.1016/0021-9681(70)90047-0. [DOI] [PubMed] [Google Scholar]
- Locniskar M., Zumla A., Mudd D. W., Isenberg D. A., Williams W., McAdam K. P. Human monoclonal antibodies to phenolic glycolipid-I derived from patients with leprosy, and production of specific anti-idiotypes. Immunology. 1988 Jun;64(2):245–251. [PMC free article] [PubMed] [Google Scholar]
- Mackworth-Young C., Sabbaga J., Schwartz R. S. Idiotypic markers of polyclonal B cell activation. Public idiotypes shared by monoclonal antibodies derived from patients with systemic lupus erythematosus or leprosy. J Clin Invest. 1987 Feb;79(2):572–581. doi: 10.1172/JCI112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masala C., Amendolea M. A., Nuti M., Riccarducci R., Tarabini C. G., Tarabini C. G. Autoantibodies in leprosy. Int J Lepr Other Mycobact Dis. 1979 Jun;47(2):171–175. [PubMed] [Google Scholar]
- Matthews L. J., Trautman J. R. Clinical and serological profiles in leprosy. Lancet. 1965 Nov 6;2(7419):915–917. doi: 10.1016/s0140-6736(65)92899-0. [DOI] [PubMed] [Google Scholar]
- Mwatha J., Moreno C., Sengupta U., Sinha S., Ivanyi J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr Rev. 1988 Sep;59(3):195–199. doi: 10.5935/0305-7518.19880024. [DOI] [PubMed] [Google Scholar]
- OLIVEIRADEALMEIDA J., BRANDAO H., GARCIADELIMA E. ENHANCED SEROLOGIC RESPONSE OF LEPROMATOUS PATIENTS TO ANTITYPHOID VACCINATION. Int J Lepr. 1964 Jul-Sep;32:292–296. [PubMed] [Google Scholar]
- Rawlinson W. D., Basten A. Cross-reactivity of Mycobacterium leprae and M. tuberculosis and the implications for assessment of in vitro T cell function in leprosy patients. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):557–559. doi: 10.1016/0035-9203(89)90294-0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Sela O., el-Roeiy A., Isenberg D. A., Kennedy R. C., Colaco C. B., Pinkhas J., Shoenfeld Y. A common anti-DNA idiotype in sera of patients with active pulmonary tuberculosis. Arthritis Rheum. 1987 Jan;30(1):50–56. doi: 10.1002/art.1780300107. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Vilner Y., Coates A. R., Rauch J., Lavie G., Shaul D., Pinkhas J. Monoclonal anti-tuberculosis antibodies react with DNA, and monoclonal anti-DNA autoantibodies react with Mycobacterium tuberculosis. Clin Exp Immunol. 1986 Nov;66(2):255–261. [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Sengupta U., Ramu G., Ivanyi J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int J Lepr Other Mycobact Dis. 1985 Mar;53(1):33–38. [PubMed] [Google Scholar]
- Strausbauch P. H., Tarrab R., Sulica A., Sela M. Stimulation in vivo and in vitro of anti-dinitrophenyl antibodies with homologous protein carriers devoid of hapten. J Immunol. 1972 Jan;108(1):236–245. [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., Schild G. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. II. Original antigenic sin: a bone marrow-derived lymphocyte memory phenomenon modulated by thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1571–1578. doi: 10.1084/jem.140.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsaae A., Ljungqvist L., Hasløv K., Heron I., Bennedsen J. Allergenic and blastogenic reactivity of three antigens from Mycobacterium tuberculosis in sensitized guinea pigs. Infect Immun. 1987 Dec;55(12):2922–2927. doi: 10.1128/iai.55.12.2922-2927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]