Abstract
A soluble form of CD23 (sCD23) was found in the urine from 12 normal individuals but was not present in 20 normal sera, suggesting that sCD23 produced by cells in tissues is eliminated in the urine. The sCD23 from urine differed in physicochemical properties from the sCD23 found in supernates from B-lymphoblastoid cell lines (B-LCL) and in the sera of patients with B type chronic lymphocytic leukaemia (B-CLL). On SDS-PAGE analysis under reducing conditions urinary sCD23 showed two bands corresponding to molecular weights of 45-60 kD and 28-35 kD indicating that sCD23 may be excreted in combination with another molecule. When subjected to gel filtration in its native state, sCD23 from urine showed a major peak at approximately 150 kD and a minor peak (probably a breakdown product) at 21 kD. Urinary sCD23 was more strongly held by DEAE-cellulose and required 0.5 M buffer pH 8.0 for elution, suggesting that it is more anionic than sCD23 from culture supernates. Five MoAbs recognizing different epitopes on sCD23 from B-LCL supernates were tested on urinary sCD23. Four of the MoAbs were reactive but one (EBVCS-1) was not. Urinary sCD23 did not bind to IgE. The level of sCD23 found in normal urine (approximately 0.02-0.05 micrograms/ml) was exceeded in 17 of 24 cases of B-CLL. In one case with a high cell count and a serum concentration of 10 micrograms/ml, the urine contained 80 micrograms/ml sCD23. In another case a high serum sCD23 was not matched by a high urinary level. In this case the gel filtration pattern was closer to that found with urine sCD23 rather than the B-LCL pattern found with sera of other B-CLL patients.
Full text
PDF

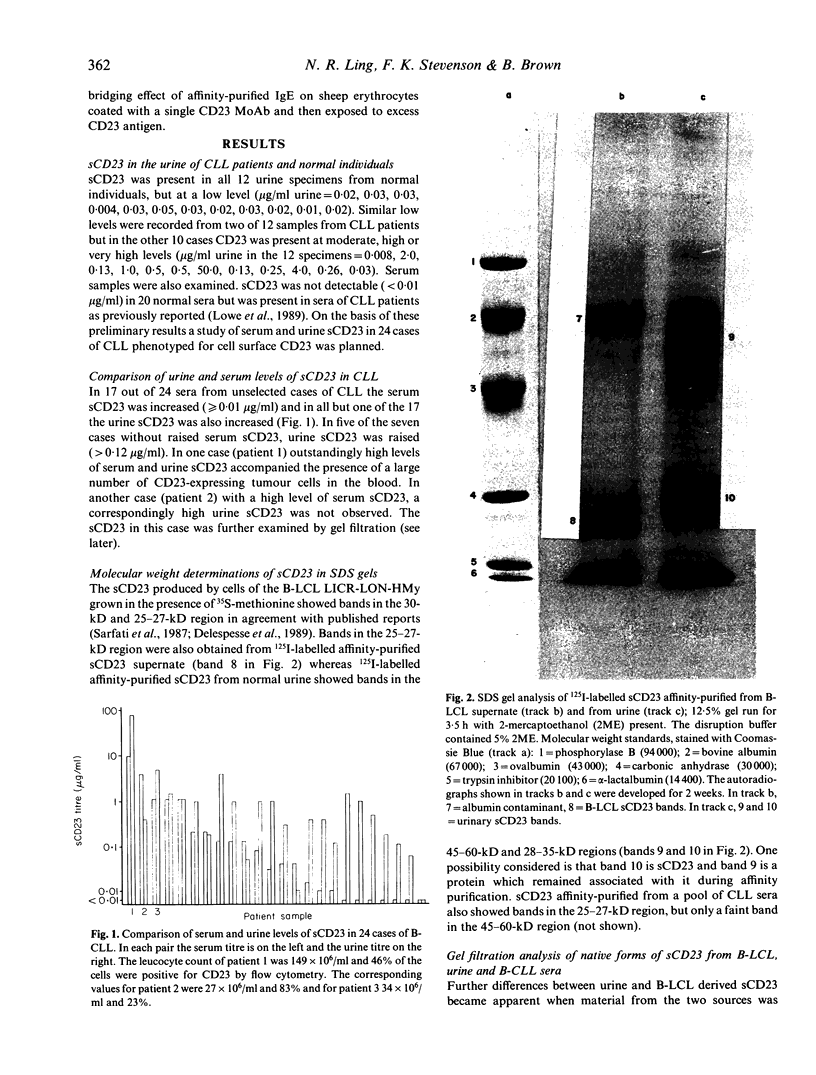


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnefoy J. Y., Defrance T., Peronne C., Menetrier C., Rousset F., Pène J., De Vries J. E., Banchereau J. Human recombinant interleukin 4 induces normal B cells to produce soluble CD23/IgE-binding factor analogous to that spontaneously released by lymphoblastoid B cell lines. Eur J Immunol. 1988 Jan;18(1):117–122. doi: 10.1002/eji.1830180118. [DOI] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Guillot O., Spits H., Blanchard D., Ishizaka K., Banchereau J. The low-affinity receptor for IgE (CD23) on B lymphocytes is spatially associated with HLA-DR antigens. J Exp Med. 1988 Jan 1;167(1):57–72. doi: 10.1084/jem.167.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespesse G., Sarfati M., Hofstetter H. Human IgE-binding factors. Immunol Today. 1989 May;10(5):159–164. doi: 10.1016/0167-5699(89)90173-4. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Aderka D., Rubinstein M., Rotman D., Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989 Jul 15;264(20):11974–11980. [PubMed] [Google Scholar]
- Gordon J., Cairns J. A., Flores-Romo L., Millsum M. J., Guy G. R. Significance of soluble CD23 ('IgE-binding factor') in pathologic sera. Blood. 1988 Jul;72(1):367–369. [PubMed] [Google Scholar]
- Gordon J., Flores-Romo L., Cairns J. A., Millsum M. J., Lane P. J., Johnson G. D., MacLennan I. C. CD23: a multi-functional receptor/lymphokine? Immunol Today. 1989 May;10(5):153–157. doi: 10.1016/0167-5699(89)90171-0. [DOI] [PubMed] [Google Scholar]
- Kintner C., Sugden B. Identification of antigenic determinants unique to the surfaces of cells transformed by Epstein-Barr virus. Nature. 1981 Dec 3;294(5840):458–460. doi: 10.1038/294458a0. [DOI] [PubMed] [Google Scholar]
- Ling N. R., Bishop S., Jefferis Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods. 1977;15(3):279–289. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- Ling N. R., Hardie D., Lowe J., Johnson G. D., Khan M., MacLennan I. C. A phenotypic study of cells from Burkitt lymphoma and EBV-B-lymphoblastoid lines and their relationship to cells in normal lymphoid tissues. Int J Cancer. 1989 Jan 15;43(1):112–118. doi: 10.1002/ijc.2910430122. [DOI] [PubMed] [Google Scholar]
- Ling N., Hansel T., Richardson P., Brown B. Cellular origins of serum complement receptor type 2 in normal individuals and in hypogammaglobulinaemia. Clin Exp Immunol. 1991 Apr;84(1):16–22. doi: 10.1111/j.1365-2249.1991.tb08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Brown B., Hardie D., Richardson P., Ling N. Soluble forms of CD21 and CD23 antigens in the serum in B cell chronic lymphocytic leukaemia. Immunol Lett. 1989 Jan 31;20(2):103–109. doi: 10.1016/0165-2478(89)90093-x. [DOI] [PubMed] [Google Scholar]
- Marcon L., Fritz M. E., Kurman C. C., Jensen J. C., Nelson D. L. Soluble Tac peptide is present in the urine of normal individuals and at elevated levels in patients with adult T cell leukaemia (ATL). Clin Exp Immunol. 1988 Jul;73(1):29–33. [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Mitchell E. B., Jouhal S. S., Flanagan J. G., Rabbitts T. H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985 Mar 21;314(6008):268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- Novick D., Engelmann H., Wallach D., Rubinstein M. Soluble cytokine receptors are present in normal human urine. J Exp Med. 1989 Oct 1;170(4):1409–1414. doi: 10.1084/jem.170.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Bron D., Lagneaux L., Fonteyn C., Frost H., Delespesse G. Elevation of IgE-binding factors in serum of patients with B cell-derived chronic lymphocytic leukemia. Blood. 1988 Jan;71(1):94–98. [PubMed] [Google Scholar]
- Sarfati M., Nakajima T., Frost H., Kilccherr E., Delespesse G. Purification and partial biochemical characterization of IgE-binding factors secreted by a human B lymphoblastoid cell line. Immunology. 1987 Apr;60(4):539–545. [PMC free article] [PubMed] [Google Scholar]