Abstract
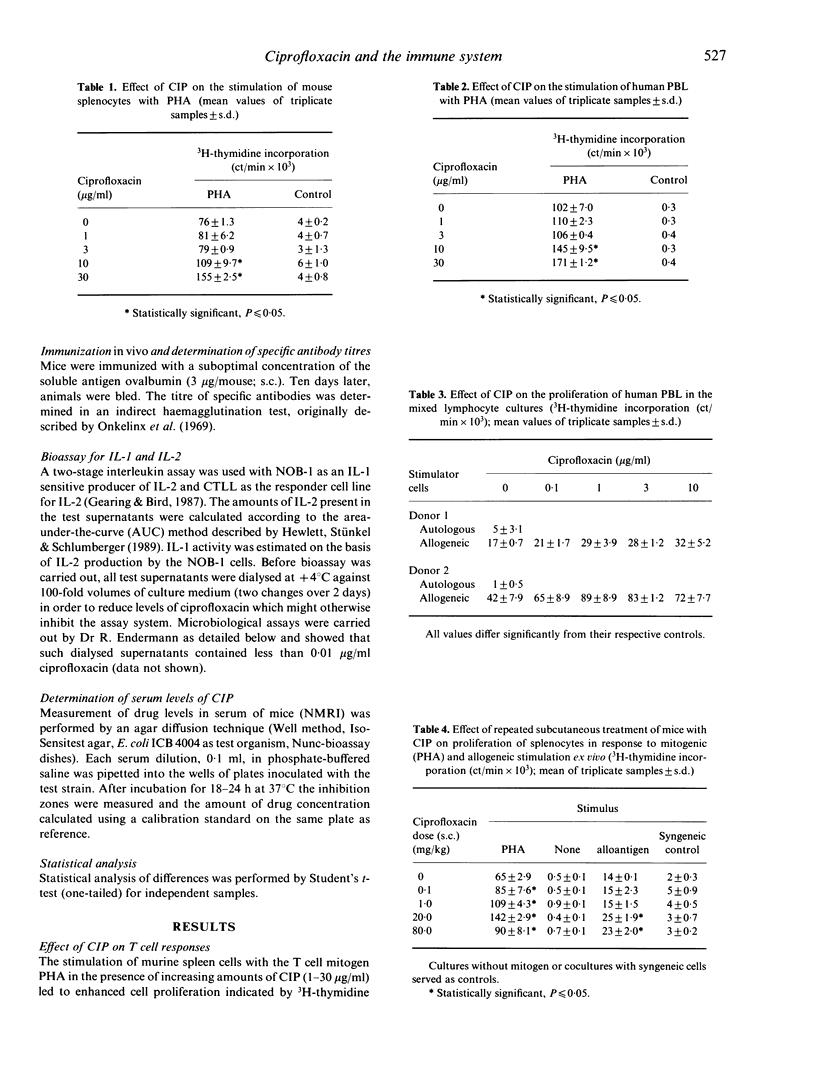
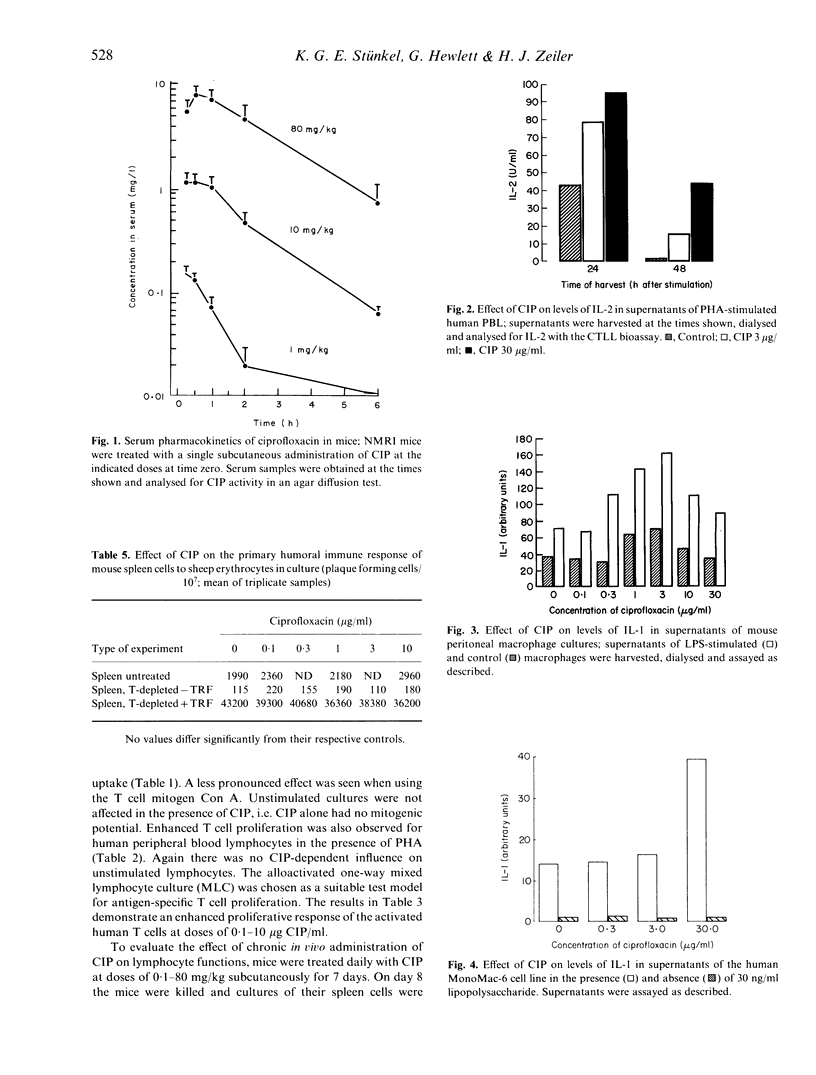
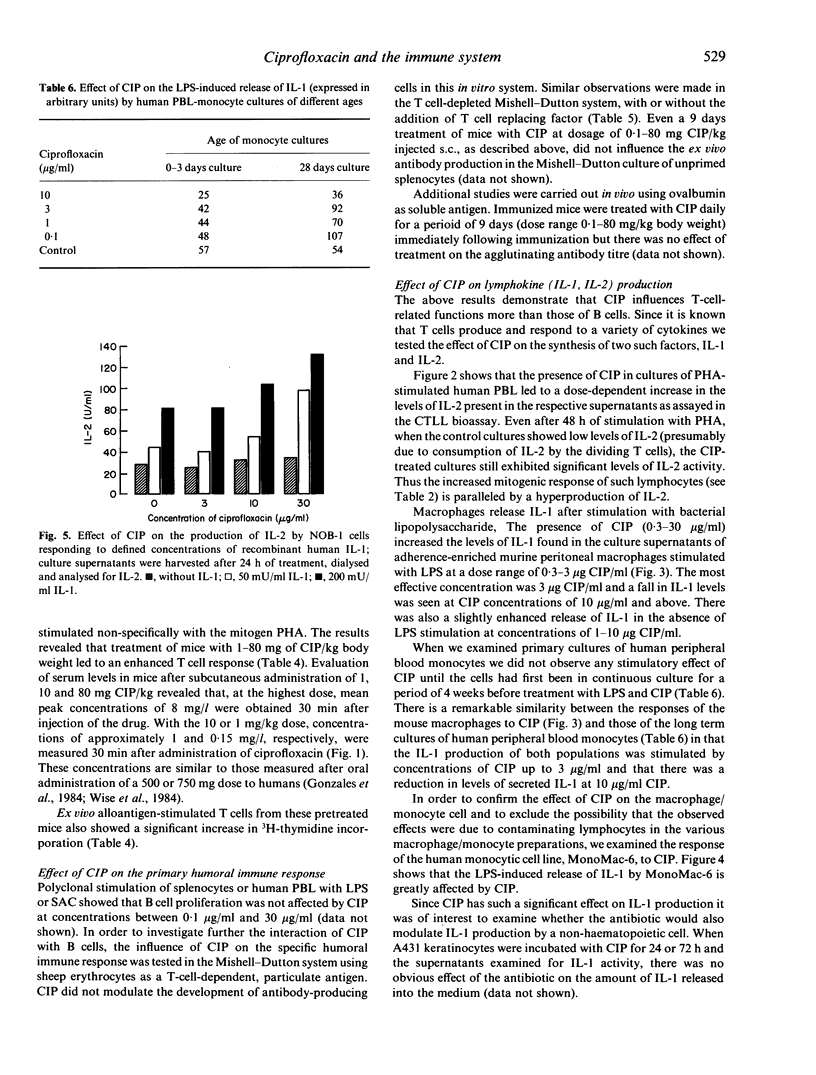
Ciprofloxacin (CIP) is a quinolone carboxylic acid derivative with a broad spectrum of antibacterial activity. CIP (0.1-30 micrograms/ml) enhanced DNA synthesis of mouse spleen cells and human peripheral blood lymphocytes (PBL) that had been activated with T cell mitogens or with alloantigens. In addition, CIP increased the amount of IL-2 found in the supernatants of phytohaemagglutinin (PHA)-stimulated human PBL. The presence of CIP in the medium (0.3-10 micrograms/ml) increased the levels of IL-1 found in the culture supernatants of adherence-enriched mouse macrophages, human monocyte/macrophages and a human monocytic cell line stimulated with lipopolysaccharide. In contrast there was no effect of CIP on the release of IL-1 by freshly isolated human monocytes or by cells of the keratinocyte line, A431. CIP alone had no influence on the basal release of IL-2 by NOB-1 cells, a T cell line that responds to IL-1 with an increase in IL-2 synthesis, but, in combination with recombinant IL-1, CIP significantly enhanced the release of IL-2 by these cells. The results of this study suggest that CIP modulates the immune response at two levels--the production of IL-2 by activated T cells and the production of IL-1 by activated monocyte/macrophages. However, CIP did not affect the primary antibody response in vitro or in vivo against sheep erythrocytes and ovalbumin respectively. Thus the enhancing action of ciprofloxacin on the immune system appears to be restricted to T cell function and macrophage/T cell interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino D., Bonina L., Berlinghieri M. C., Mastroeni P. Effects of a new quinoline derivative, ciprofloxacin, on some professional phagocytic cell functions. Chemioterapia. 1985 Dec;4(6):463–466. [PubMed] [Google Scholar]
- Eisen S. A., Wedner H. J., Parker C. W. Isolation of pure human peripheral blood T-lymphocytes using nylon wool columns. Immunol Commun. 1972;1(6):571–577. doi: 10.3109/08820137209022965. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Gollapudi S. V., Prabhala R. H., Thadepalli H. Effect of ciprofloxacin on mitogen-stimulated lymphocyte proliferation. Antimicrob Agents Chemother. 1986 Feb;29(2):337–338. doi: 10.1128/aac.29.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Uribe F., Moisen S. D., Fuster A. P., Selen A., Welling P. G., Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Remington J. S. Effect of antibiotics on the immune response. Am J Med. 1982 May;72(5):711–716. doi: 10.1016/0002-9343(82)90534-4. [DOI] [PubMed] [Google Scholar]
- Hewlett G., Stünkel K. G., Schlumberger H. D. A method for the quantitation of interleukin-2 activity. J Immunol Methods. 1989 Feb 24;117(2):243–246. doi: 10.1016/0022-1759(89)90146-4. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Luger T. A., Köck A., Danner M. Characterization of immunoregulatory cytokines produced by epidermal cells. Scand J Immunol. 1985 May;21(5):455–462. doi: 10.1111/j.1365-3083.1985.tb01832.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munker R., Stünkel K., Thiel E., Thierfelder S. Analysis with monoclonal antibodies of human lymphoid cells forming rosettes with rabbit red blood cells. Clin Exp Immunol. 1983 Mar;51(3):479–486. [PMC free article] [PubMed] [Google Scholar]
- Onkelinx E., Meuldermans W., Joniau M., Lontie R. Glutaraldehyde as a coupling reagent in passive haemagglutination. Immunology. 1969 Jan;16(1):35–43. [PMC free article] [PubMed] [Google Scholar]
- Opitz H. G., Opitz U., Hewlett G., Schlumberger H. D. A new model for investigations of T-cell functions in mice: differential immunosuppressive effects of two monoclonal anti-Thy-1.2 antibodies. Immunobiology. 1982 Feb;160(5):438–453. doi: 10.1016/S0171-2985(82)80007-7. [DOI] [PubMed] [Google Scholar]
- Oschilewski M., Schwab E., Kiesel U., Opitz U., Stünkel K., Kolb-Bachofen V., Kolb H. Administration of silica or monoclonal antibody to Thy-1 prevents low-dose streptozotocin-induced diabetes in mice. Immunol Lett. 1986 Jun;12(5-6):289–294. doi: 10.1016/0165-2478(86)90032-5. [DOI] [PubMed] [Google Scholar]
- Potts R. C., Hassan H. A., Brown R. A., MacConnachie A., Gibbs J. H., Robertson A. J., Beck J. S. In vitro effects of doxycycline and tetracycline on mitogen stimulated lymphocyte growth. Clin Exp Immunol. 1983 Aug;53(2):458–464. [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Riesbeck K., Andersson J., Gullberg M., Forsgren A. Fluorinated 4-quinolones induce hyperproduction of interleukin 2. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2809–2813. doi: 10.1073/pnas.86.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche Y., Fay M., Gougerot-Pocidalo M. A. Effects of quinolones on interleukin 1 production in vitro by human monocytes. Immunopharmacology. 1987 Apr;13(2):99–109. doi: 10.1016/0162-3109(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Roche Y., Fay M., Gougerot-Pocidalo M. A. Enhancement of interleukin 2 production by quinolone-treated human mononuclear leukocytes. Int J Immunopharmacol. 1988;10(2):161–167. doi: 10.1016/0192-0561(88)90091-4. [DOI] [PubMed] [Google Scholar]
- Roche Y., Gougerot-Pocidalo M. A., Fay M., Etienne D., Forest N., Pocidalo J. J. Comparative effects of quinolones on human mononuclear leucocyte functions. J Antimicrob Chemother. 1987 Jun;19(6):781–790. doi: 10.1093/jac/19.6.781. [DOI] [PubMed] [Google Scholar]
- Roszkowski W., Ko H. L., Roszkowski K., Jeljaszewicz J., Pulverer G. Antibiotics and immunomodulation: effects of cefotaxime, amikacin, mezlocillin, piperacillin and clindamycin. Med Microbiol Immunol. 1985;173(5):279–289. doi: 10.1007/BF02124944. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]


