Abstract
IL-6, an important mediator of the acute phase response, has been implicated in the pathogenesis of rheumatoid arthritis (RA). Many cell types including macrophages, T cells, B cells, endothelial cells and fibroblasts can produce this cytokine and production is largely regulated at the level of gene transcription or mRNA stabilization. In this paper we have first measured the levels of IL-6 activity in synovial fluid (SF) and serum from patients with RA and then localized IL-6-producing cells in the synovium by in situ hybridization combined with immunophenotyping. Patients with RA had raised levels of IL-6 in both SF and serum compared with patients with osteoarthritis and age-matched healthy controls. In individual RA patients tested serially after admission to hospital, serum IL-6 was initially raised and, unexpectedly, increased with clinical improvement. In situ hybridization of IL-6 mRNA showed positive cells both in the lymphocyte-rich aggregates and adjacent to small blood vessels. With immunophenotyping it was found that cells containing IL-6 mRNA were often in contact with CD14+ tissue macrophages and double immunophenotyping revealed that immunoreactive IL-6 was often associated with synovial T cells.
Full text
PDF

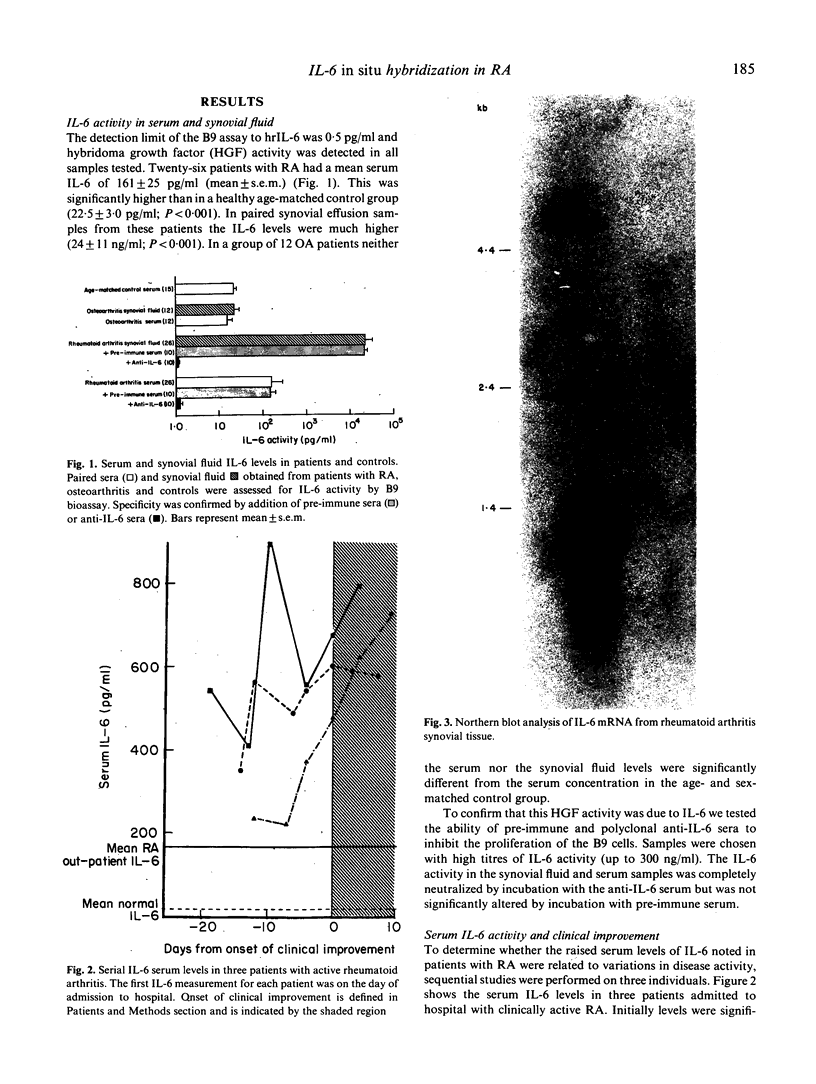
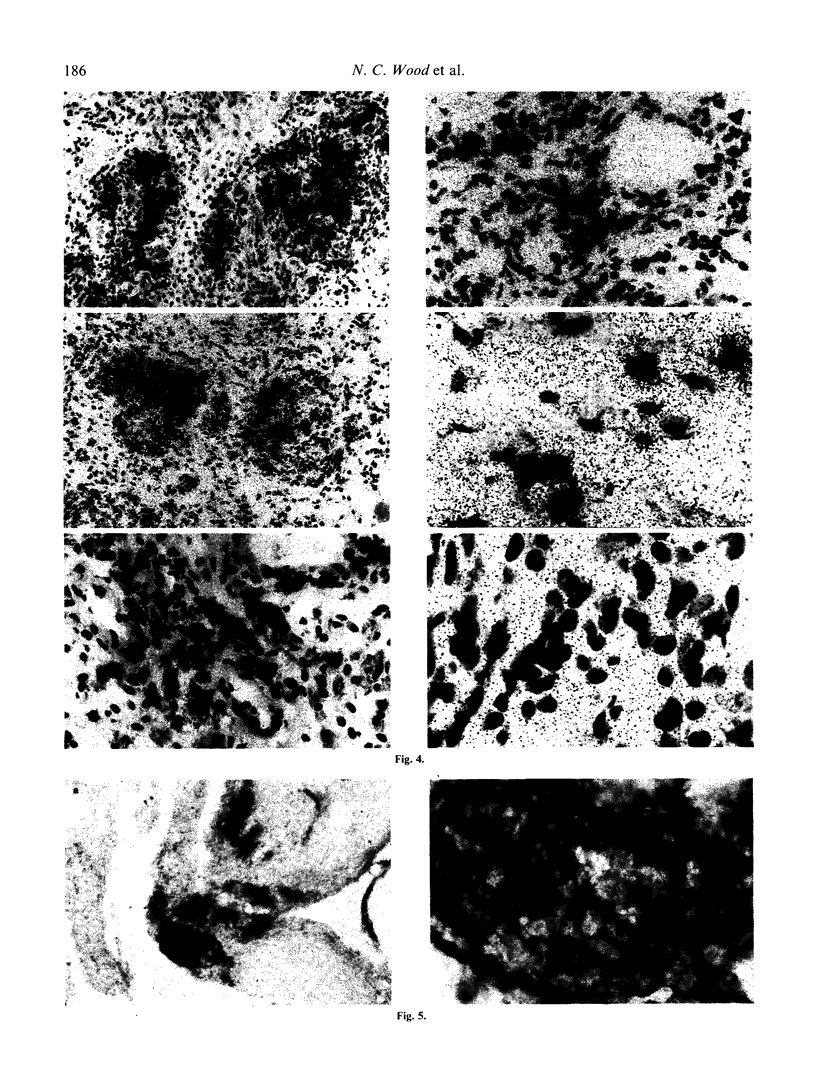



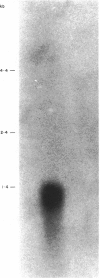
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Astaldi G. C., Janssen M. C., Lansdorp P., Willems C., Zeijlemaker W. P., Oosterhof F. Human endothelial culture supernatant (HECS): a growth factor for hybridomas. J Immunol. 1980 Oct;125(4):1411–1414. [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L., Lorre K., Van Damme J., Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 1988 Dec 1;141(11):3868–3874. [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Fehr K., Velvart M., Rauber M., Knöpfel M., Baici A., Salgam P., Böni A. Production of agglutinators and rheumatoid factors in plasma cells of rheumatoid and nonrheumatoid synovial tissues. Arthritis Rheum. 1981 Mar;24(3):510–519. doi: 10.1002/art.1780240310. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Content J., Volckaert G., Derynck R., Tavernier J., Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986 Sep 15;159(3):625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Hirano T., Taga T., Yasukawa K., Nakajima K., Nakano N., Takatsuki F., Shimizu M., Murashima A., Tsunasawa S., Sakiyama F. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A. 1987 Jan;84(1):228–231. doi: 10.1073/pnas.84.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Horii Y., Muraguchi A., Suematsu S., Matsuda T., Yoshizaki K., Hirano T., Kishimoto T. Regulation of BSF-2/IL-6 production by human mononuclear cells. Macrophage-dependent synthesis of BSF-2/IL-6 by T cells. J Immunol. 1988 Sep 1;141(5):1529–1535. [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Kurosaka M., Ziff M. Electron microscopic study of HLA-DR and monocyte/macrophage staining cells in the rheumatoid synovial membrane. Arthritis Rheum. 1986 May;29(5):600–613. doi: 10.1002/art.1780290504. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T., Mizuta T., Rosén A., Hirano T., Kishimoto T., Honjo T. Enhancement of the interleukin 2 receptor expression on T cells by multiple B-lymphotropic lymphokines. Immunol Lett. 1987 Jul;15(3):249–253. doi: 10.1016/0165-2478(87)90032-0. [DOI] [PubMed] [Google Scholar]
- Ogilvie A. D., Wood N. C., Dickens E., Wojtacha D., Duff G. W. In situ hybridisation. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):434–439. [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Swaak A. J., van Rooyen A., Nieuwenhuis E., Aarden L. A. Interleukin-6 (IL-6) in synovial fluid and serum of patients with rheumatic diseases. Scand J Rheumatol. 1988;17(6):469–474. doi: 10.3109/03009748809098809. [DOI] [PubMed] [Google Scholar]
- Symons J. A., McDowell T. L., di Giovine F. S., Wood N. C., Capper S. J., Duff G. W. Interleukin 1 in rheumatoid arthritis: potentiation of immune responses within the joint. Lymphokine Res. 1989 Fall;8(3):365–372. [PubMed] [Google Scholar]
- Van Damme J., Cayphas S., Opdenakker G., Billiau A., Van Snick J. Interleukin 1 and poly(rI).poly(rC) induce production of a hybridoma growth factor by human fibroblasts. Eur J Immunol. 1987 Jan;17(1):1–7. doi: 10.1002/eji.1830170102. [DOI] [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Decock B., Van Snick J., De Ley M., Billiau A. Separation and comparison of two monokines with lymphocyte-activating factor activity: IL-1 beta and hybridoma growth factor (HGF). Identification of leukocyte-derived HGF as IL-6. J Immunol. 1988 Mar 1;140(5):1534–1541. [PubMed] [Google Scholar]
- Wernick R. M., Lipsky P. E., Marban-Arcos E., Maliakkal J. J., Edelbaum D., Ziff M. IgG and IgM rheumatoid factor synthesis in rheumatoid synovial membrane cell cultures. Arthritis Rheum. 1985 Jul;28(7):742–752. doi: 10.1002/art.1780280704. [DOI] [PubMed] [Google Scholar]
- Wood N. C., Symons J. A., Duff G. W. Serum interleukin-2-receptor in rheumatoid arthritis: a prognostic indicator of disease activity? J Autoimmun. 1988 Aug;1(4):353–361. doi: 10.1016/0896-8411(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giovine F. S., Malawista S. E., Thornton E., Duff G. W. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J Clin Invest. 1991 Apr;87(4):1375–1381. doi: 10.1172/JCI115142. [DOI] [PMC free article] [PubMed] [Google Scholar]