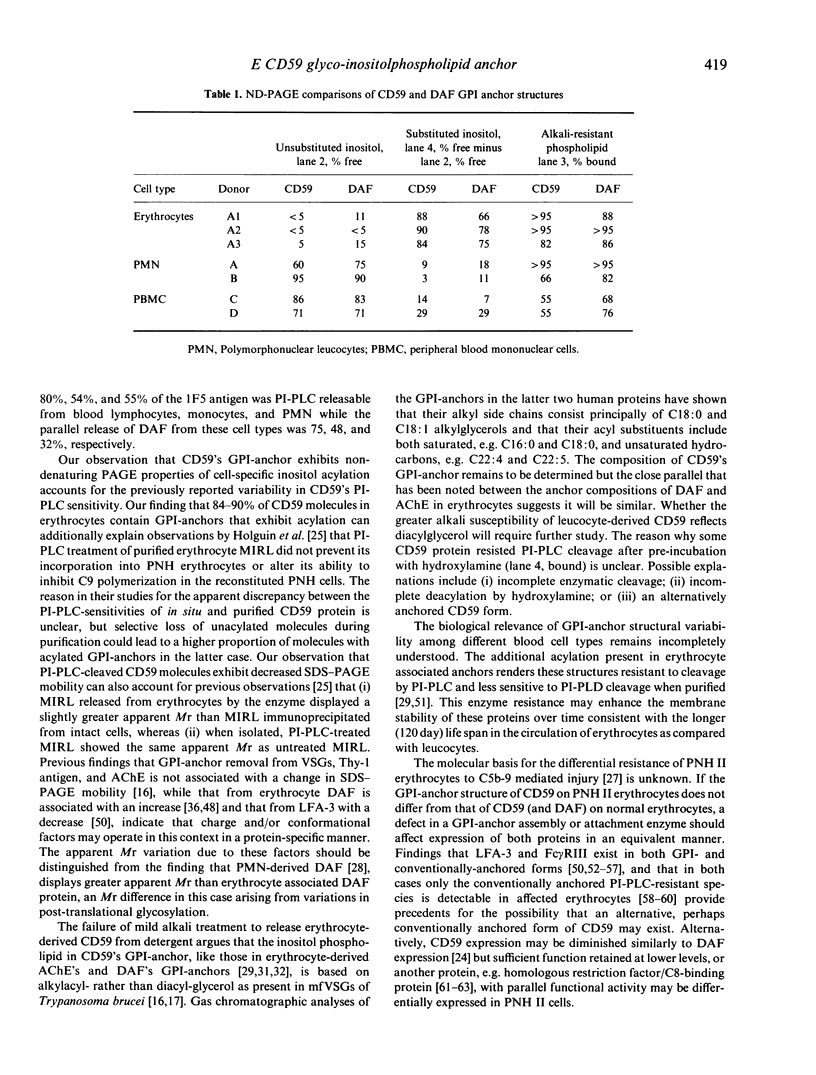
Abstract
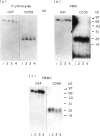
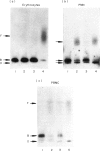
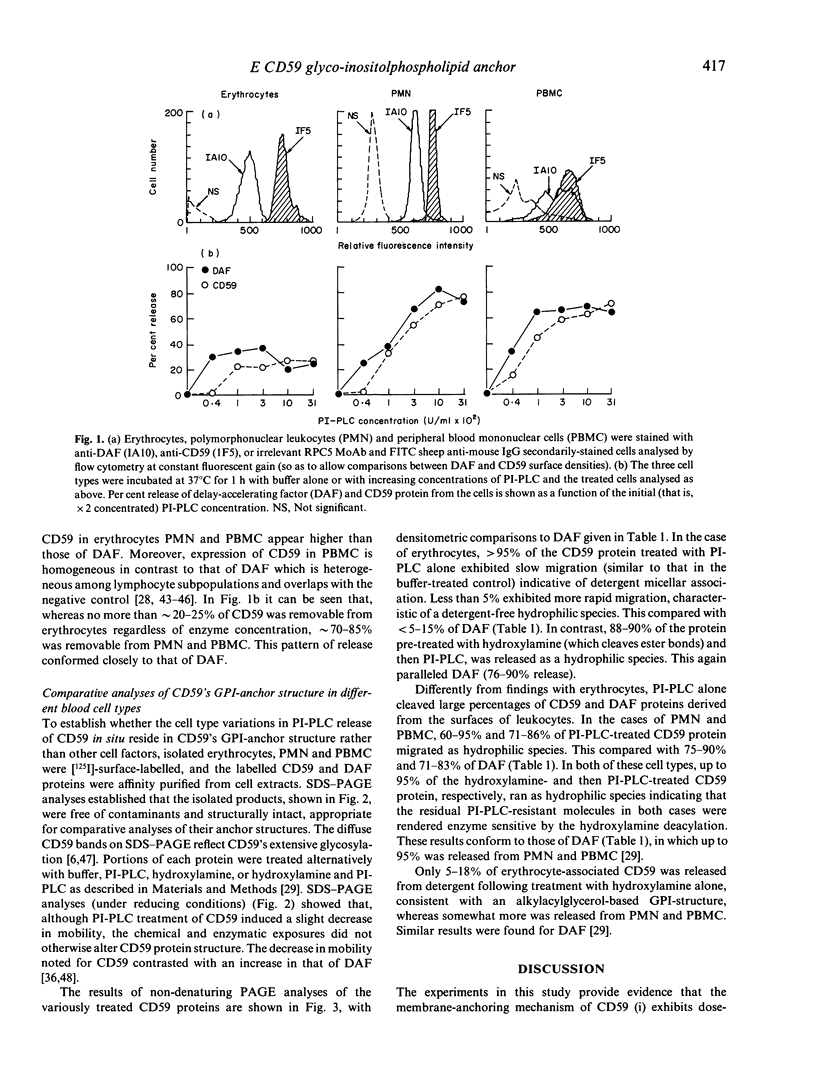
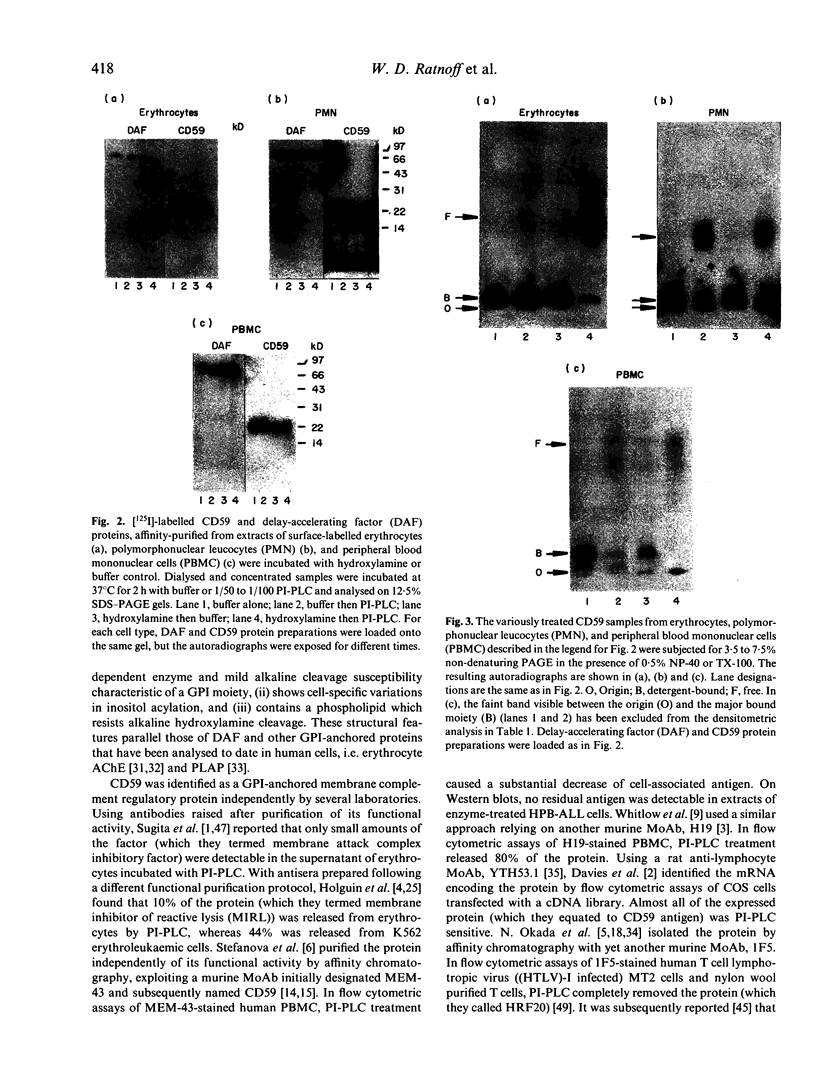
CD59, the membrane regulator of autologous C5b-9 channel formation, exhibits variable sensitivity to cleavage by phosphatidylinositol-specific phospholipase C (PI-PLC), an enzyme that releases glyco-inositolphospholipid (GPI)-anchored proteins from cell surfaces. To determine whether the GPI-anchor phospholipid of CD59 is similar to that of decay-accelerating factor (DAF) and whether variation in its structure underlies its variable enzyme susceptibility, the GPI anchors of the two proteins expressed on erythrocytes, polymorphonuclear and mononuclear leucocytes were compared in situ and after purification. Flow cytometric analyses of PI-PLC-treated cells showed parallel cell type specific release of both proteins as a function of enzyme concentration. Non-denaturing PAGE analyses of alkaline/hydroxylamine-treated proteins (affinity-purified from [125I]-surface-labelled cells) provided evidence for (i) comparable proportions of GPI-anchor acylation, and (ii) alkali-resistant rather than alkali-sensitive lipid substituents in erythrocytes. These findings argue that the differential C5b-9 sensitivity that distinguishes paroxysmal nocturnal haemoglobinuria II and III erythrocytes does not derive from expression of CD59 molecules with alternative GPI-anchor phospholipid structures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger M., Medof M. E. Increased expression of complement decay-accelerating factor during activation of human neutrophils. J Clin Invest. 1987 Jan;79(1):214–220. doi: 10.1172/JCI112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers D. J., Hazra S. V., Andreson S. W., Medof M. E. Synthesis of aberrant decay-accelerating factor proteins by affected paroxysmal nocturnal hemoglobinuria leukocytes. J Clin Invest. 1990 Jan;85(1):47–54. doi: 10.1172/JCI114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Selvaraj P., Mattaliano R. J., Springer T. A. Anchoring mechanisms for LFA-3 cell adhesion glycoprotein at membrane surface. 1987 Oct 29-Nov 4Nature. 329(6142):846–848. doi: 10.1038/329846a0. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Groux H., Huet S., Aubrit F., Tran H. C., Boumsell L., Bernard A. A 19-kDa human erythrocyte molecule H19 is involved in rosettes, present on nucleated cells, and required for T cell activation. Comparison of the roles of H19 and LFA-3 molecules in T cell activation. J Immunol. 1989 May 1;142(9):3013–3020. [PubMed] [Google Scholar]
- Hale G., Bright S., Chumbley G., Hoang T., Metcalf D., Munro A. J., Waldmann H. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood. 1983 Oct;62(4):873–882. [PubMed] [Google Scholar]
- Hammelburger J. W., Palfree R. G., Sirlin S., Hämmerling U. Demonstration of phosphatidylinositol anchors on Ly-6 molecules by specific phospholipase C digestion and gel electrophoresis in octylglucoside. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1304–1311. doi: 10.1016/s0006-291x(87)80275-9. [DOI] [PubMed] [Google Scholar]
- Hideshima T., Okada N., Okada H. Expression of HRF20, a regulatory molecule of complement activation, on peripheral blood mononuclear cells. Immunology. 1990 Mar;69(3):396–401. [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. M. Inhibition of complement by a substance isolated from human erythrocytes. I. Extraction from human erythrocyte stromata. Immunochemistry. 1969 May;6(3):391–403. doi: 10.1016/0019-2791(69)90296-1. [DOI] [PubMed] [Google Scholar]
- Holguin M. H., Fredrick L. R., Bernshaw N. J., Wilcox L. A., Parker C. J. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Jul;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin M. H., Wilcox L. A., Bernshaw N. J., Rosse W. F., Parker C. J. Erythrocyte membrane inhibitor of reactive lysis: effects of phosphatidylinositol-specific phospholipase C on the isolated and cell-associated protein. Blood. 1990 Jan 1;75(1):284–289. [PubMed] [Google Scholar]
- Holguin M. H., Wilcox L. A., Bernshaw N. J., Rosse W. F., Parker C. J. Relationship between the membrane inhibitor of reactive lysis and the erythrocyte phenotypes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Nov;84(5):1387–1394. doi: 10.1172/JCI114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga T. W., van der Schoot C. E., Jost C., Klaassen R., Kleijer M., von dem Borne A. E., Roos D., Tetteroo P. A. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988 Jun 16;333(6174):667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Gottlieb A., Kinoshita T., Hall S., Silber R., Nussenzweig V., Rosse W. F. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987 Jul;80(1):165–174. doi: 10.1172/JCI113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Wing M., Jones J., Davies A., Podack E., Lachmann P. J. Human protectin (CD59), an 18-20-kD homologous complement restriction factor, does not restrict perforin-mediated lysis. J Exp Med. 1990 Jul 1;172(1):367–370. doi: 10.1084/jem.172.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Nicholson-Weller A., Russian D. A., Austen K. F. Natural killer cells are deficient in the surface expression of the complement regulatory protein, decay accelerating factor (DAF). J Immunol. 1986 Aug 15;137(4):1275–1279. [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989 Oct 1;143(7):2262–2266. [PubMed] [Google Scholar]
- Okada N., Harada R., Taguchi R., Okada H. Complete deficiency of 20 KDa homologous restriction factor (HRF20) and restoration with purified HRF20. Biochem Biophys Res Commun. 1989 Oct 16;164(1):468–473. doi: 10.1016/0006-291x(89)91743-9. [DOI] [PubMed] [Google Scholar]
- Packman C. H., Rosenfeld S. I., Jenkins D. E., Jr, Thiem P. A., Leddy J. P. Complement lysis of human erythrocytes. Differeing susceptibility of two types of paroxysmal nocturnal hemoglobinuria cells to C5b-9. J Clin Invest. 1979 Aug;64(2):428–433. doi: 10.1172/JCI109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989 Aug 1;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Roberts W. L., Santikarn S., Reinhold V. N., Rosenberry T. L. Structural characterization of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase by fast atom bombardment mass spectrometry. J Biol Chem. 1988 Dec 15;263(35):18776–18784. [PubMed] [Google Scholar]
- Rollins S. A., Sims P. J. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990 May 1;144(9):3478–3483. [PubMed] [Google Scholar]
- Rosse W. F. Phosphatidylinositol-linked proteins and paroxysmal nocturnal hemoglobinuria. Blood. 1990 Apr 15;75(8):1595–1601. [PubMed] [Google Scholar]
- Rosse W. F. Variations in the red cells in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1973 Mar;24(3):327–342. doi: 10.1111/j.1365-2141.1973.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Scallon B. J., Scigliano E., Freedman V. H., Miedel M. C., Pan Y. C., Unkeless J. C., Kochan J. P. A human immunoglobulin G receptor exists in both polypeptide-anchored and phosphatidylinositol-glycan-anchored forms. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5079–5083. doi: 10.1073/pnas.86.13.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Silber R., Low M. G., Springer T. A. Deficiency of lymphocyte function-associated antigen 3 (LFA-3) in paroxysmal nocturnal hemoglobinuria. Functional correlates and evidence for a phosphatidylinositol membrane anchor. J Exp Med. 1987 Oct 1;166(4):1011–1025. doi: 10.1084/jem.166.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Rosse W. F., Silber R., Springer T. A. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature. 1988 Jun 9;333(6173):565–567. doi: 10.1038/333565a0. [DOI] [PubMed] [Google Scholar]
- Simmons D., Seed B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature. 1988 Jun 9;333(6173):568–570. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Tykocinski M. L., Lublin D. M., Holers V. M., Rosse W. F., Atkinson J. P., Medof M. E. Normal polymorphic variations and transcription of the decay accelerating factor gene in paroxysmal nocturnal hemoglobinuria cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):880–884. doi: 10.1073/pnas.85.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Hilgert I., Kristofová H., Brown R., Low M. G., Horejsí V. Characterization of a broadly expressed human leucocyte surface antigen MEM-43 anchored in membrane through phosphatidylinositol. Mol Immunol. 1989 Feb;26(2):153–161. doi: 10.1016/0161-5890(89)90097-7. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Mazda T., Tomita M. Amino-terminal amino acid sequence and chemical and functional properties of a membrane attack complex-inhibitory factor from human erythrocyte membranes. J Biochem. 1989 Oct;106(4):589–592. doi: 10.1093/oxfordjournals.jbchem.a122900. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Funahashi Y., Ikezawa H., Nakashima I. Analysis of PI (phosphatidylinositol)-anchoring antigens in a patient of paroxysmal nocturnal hemoglobinuria (PNH) reveals deficiency of 1F5 antigen (CD59), a new complement-regulatory factor. FEBS Lett. 1990 Feb 12;261(1):142–146. doi: 10.1016/0014-5793(90)80656-4. [DOI] [PubMed] [Google Scholar]
- Takami N., Ogata S., Oda K., Misumi Y., Ikehara Y. Biosynthesis of placental alkaline phosphatase and its post-translational modification by glycophospholipid for membrane-anchoring. J Biol Chem. 1988 Feb 25;263(6):3016–3021. [PubMed] [Google Scholar]
- Toutant J. P., Roberts W. L., Murray N. R., Rosenberry T. L. Conversion of human erythrocyte acetylcholinesterase from an amphiphilic to a hydrophilic form by phosphatidylinositol-specific phospholipase C and serum phospholipase D. Eur J Biochem. 1989 Apr 1;180(3):503–508. doi: 10.1111/j.1432-1033.1989.tb14674.x. [DOI] [PubMed] [Google Scholar]
- Ueda E., Kinoshita T., Nojima J., Inoue K., Kitani T. Different membrane anchors of Fc gamma RIII (CD16) on K/NK-lymphocytes and neutrophils. Protein- vs lipid-anchor. J Immunol. 1989 Aug 15;143(4):1274–1277. [PubMed] [Google Scholar]
- Walter E. I., Roberts W. L., Rosenberry T. L., Ratnoff W. D., Medof M. E. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990 Feb 1;144(3):1030–1036. [PubMed] [Google Scholar]
- Watts M. J., Dankert J. R., Morgan E. P. Isolation and characterization of a membrane-attack-complex-inhibiting protein present in human serum and other biological fluids. Biochem J. 1990 Jan 15;265(2):471–477. doi: 10.1042/bj2650471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow M. B., Iida K., Stefanova I., Bernard A., Nussenzweig V. H19, a surface membrane molecule involved in T-cell activation, inhibits channel formation by human complement. Cell Immunol. 1990 Mar;126(1):176–184. doi: 10.1016/0008-8749(90)90310-n. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Müller-Eberhard H. J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]