Abstract
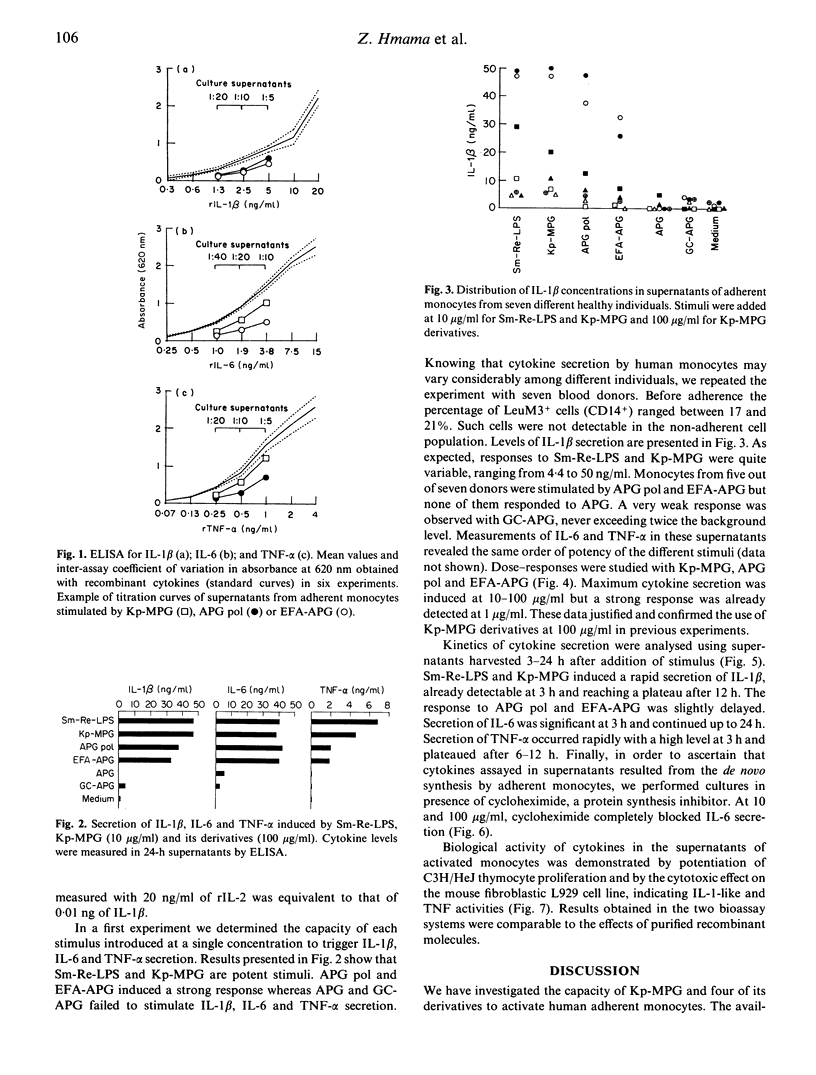
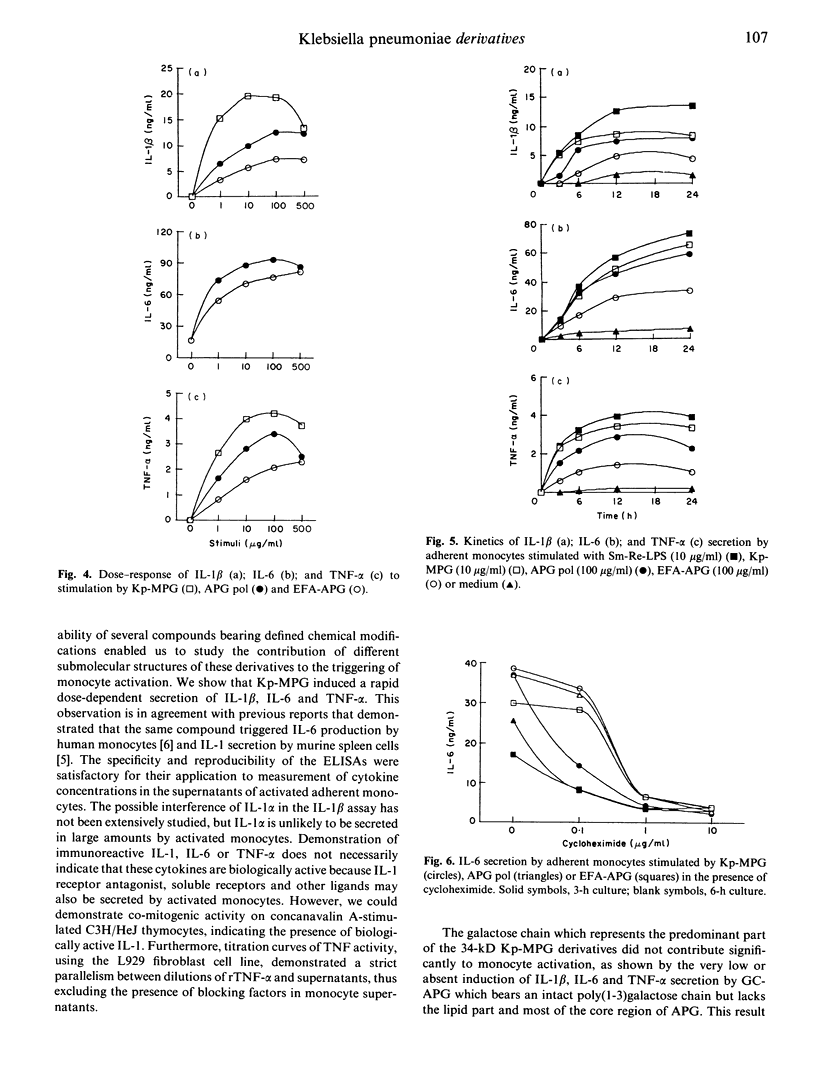
The capacity of a K. pneumoniae membrane proteoglycan (Kp-MPG) and four of its chemically defined derivatives to activate human monocytes was studied by measuring immunoreactive IL-1 beta, IL-6 and tumour necrosis factor-alpha (TNF-alpha) in culture supernatants. Monocyte culture supernatants were also tested for their comitogenic activity on concanavalin A-stimulated thymocytes and for their cytotoxic activity on the mouse fibroblastic L929 cell line. The four Kp-MPG derivatives were: (i) an acylpoly(1-3)galactoside (APG); (ii) an APG preparation submitted to acid hydrolysis which removed all fatty acids but left intact the galactose chain of APG (GC-APG); (iii) a preparation obtained by mild alkaline hydrolysis, containing additional ester-linked C14 and C16 fatty acids bound to the APG molecule (EFA-APG); and (iv) a polymer of the latter compound (APG pol). Kp-MPG induced the synthesis of IL-1 beta, IL-6 and TNF-alpha with dose-responses and kinetics similar to those of Salmonella minnesota lipopolysaccharide (Sm-Re-LPS). APG pol and EFA-APG induced the secretion of the three cytokines with lower potency than Kp-MPG or Sm-Re-LPS. APG did not trigger any detectable cytokine production and GC-APG induced only borderline and inconsistent responses. Our data demonstrate the critical role of ester-linked C14 and C16 fatty acids in the triggering of monocyte response to Kp-MPG derivatives.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dijkstra J., Larrick J. W., Ryan J. L., Szoka F. C. Incorporation of LPS in liposomes diminishes its ability to induce tumoricidal activity and tumor necrosis factor secretion in murine macrophages. J Leukoc Biol. 1988 May;43(5):436–444. doi: 10.1002/jlb.43.5.436. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Caroff M., Cavaillon J. M. Interleukin-1 induction by lipopolysaccharides: structural requirements of the 3-deoxy-D-manno-2-octulosonic acid (KDO). Mol Immunol. 1989 May;26(5):485–494. doi: 10.1016/0161-5890(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Lasfargues A., Ledur A., Charon D., Szabo L., Chaby R. Induction by lipopolysaccharide of intracellular and extracellular interleukin 1 production: analysis with synthetic models. J Immunol. 1987 Jul 15;139(2):429–436. [PubMed] [Google Scholar]
- Lebbar S., Cavaillon J. M., Caroff M., Ledur A., Brade H., Sarfati R., Haeffner-Cavaillon N. Molecular requirement for interleukin 1 induction by lipopolysaccharide-stimulated human monocytes: involvement of the heptosyl-2-keto-3-deoxyoctulosonate region. Eur J Immunol. 1986 Jan;16(1):87–91. doi: 10.1002/eji.1830160117. [DOI] [PubMed] [Google Scholar]
- Li Y. S., Kouassi E., Revillard J. P. Differential regulation of mouse B-cell activation by beta-adrenoceptor stimulation depending on type of mitogens. Immunology. 1990 Mar;69(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Brade H., Dürrbaum I., Dinarello C. A., Kusumoto S., Rietschel E. T., Flad H. D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989 May 1;142(9):3229–3238. [PubMed] [Google Scholar]
- Loppnow H., Brade L., Brade H., Rietschel E. T., Kusumoto S., Shiba T., Flad H. D. Induction of human interleukin 1 by bacterial and synthetic lipid A. Eur J Immunol. 1986 Oct;16(10):1263–1267. doi: 10.1002/eji.1830161013. [DOI] [PubMed] [Google Scholar]
- Michel F. B., Dussourd D'Hinterland L., Bousquet J., Pinel A. M., Normier G. Immuno-stimulation by a ribosomal vaccine associated with a bacterial cell wall adjuvant in humans. Infect Immun. 1978 Jun;20(3):760–769. doi: 10.1128/iai.20.3.760-769.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet I., Lafont S., de Fraissinette A., Jeannin M., Revillard J. P., Normier G., Dussourd d'Hinterland L. Polyclonal activation of murine B cells by a membrane proteoglycan of Klebsiella pneumoniae. Clin Exp Immunol. 1987 Oct;70(1):201–208. [PMC free article] [PubMed] [Google Scholar]
- Normier G., Pinel A. M., Dussourd D'Hinterland L., Ramstedt U., Wigzell H. NK-cell stimulating properties of a membrane proteoglycane from non-capsulated Klebsiella pneumoniae biotype a. Acta Pathol Microbiol Immunol Scand C. 1985 Dec;93(6):233–243. doi: 10.1111/j.1699-0463.1985.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Sironi M., Sica A., Riganti F., Licciardello L., Colotta F., Mantovani A. Interleukin-6 gene expression and production induced in human monocytes by membrane proteoglycans from Klebsiella pneumoniae. Int J Immunopharmacol. 1990;12(4):397–402. doi: 10.1016/0192-0561(90)90021-e. [DOI] [PubMed] [Google Scholar]
- Wright S. D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991 Feb;3(1):83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]


