Abstract
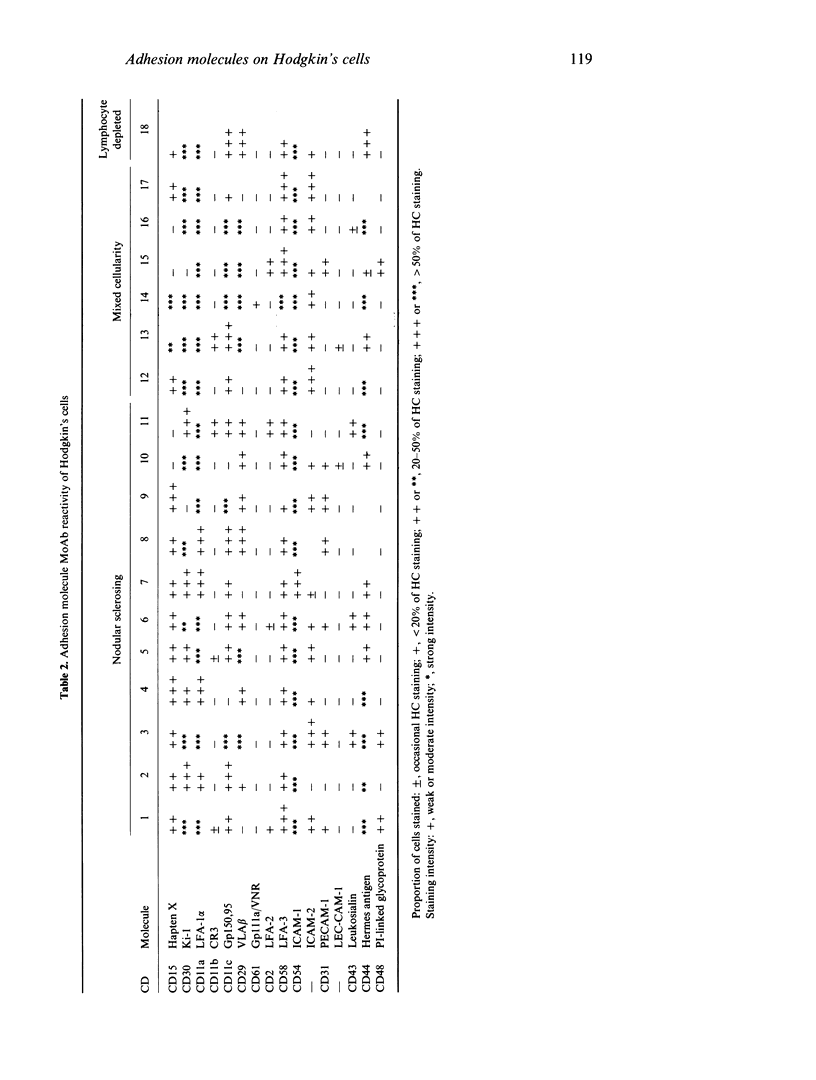
Adhesion molecules play an important role in the functioning of the immune system, particularly with regard to cell-cell interactions and antigen presentation. Several adhesion molecules are expressed on Hodgkin's disease-derived cell lines and these are important in their molecular interactions as antigen presenting cells (APC). There are no data regarding the expression of many of these adhesion molecules on Reed-Sternberg cells and its mononuclear variant (Hodgkin's cells (HC)) present in pathological material. To obtain this information we undertook an immunohistological study on material from 18 cases of Hodgkin's disease using a panel of MoAbs to examine the expression of adhesion molecules on HC. The HC were shown to express the integrin beta 1 subfamily molecules, LFA-1 (CD11a) and p150,95 (CD11c) in high density but lacked CR3 (CD11b). All of the immunoglobulin gene superfamily adhesion molecules studied were present to some degree on HC, with ICAM-2, in particular, showing moderate to strong expression in most cases. The Hermes antigen CD44 was present in high density but leukosialin (CD43), another molecule present on diverse leucocyte types, was, in general, not detected on HC. These new data showing that ICAM-1, ICAM-2 and LFA-3 are, like LFA-1, expressed on HC emphasize the ability of HC to act as APC. The known adhesion molecule phenotype of the recently defined haematopoietic lineage of human dendritic cells (DC) is broadly similar to that of HC, perhaps supporting the hypothesis that some HC represent a malignancy of an APC (DC) lineage.
Full text
PDF


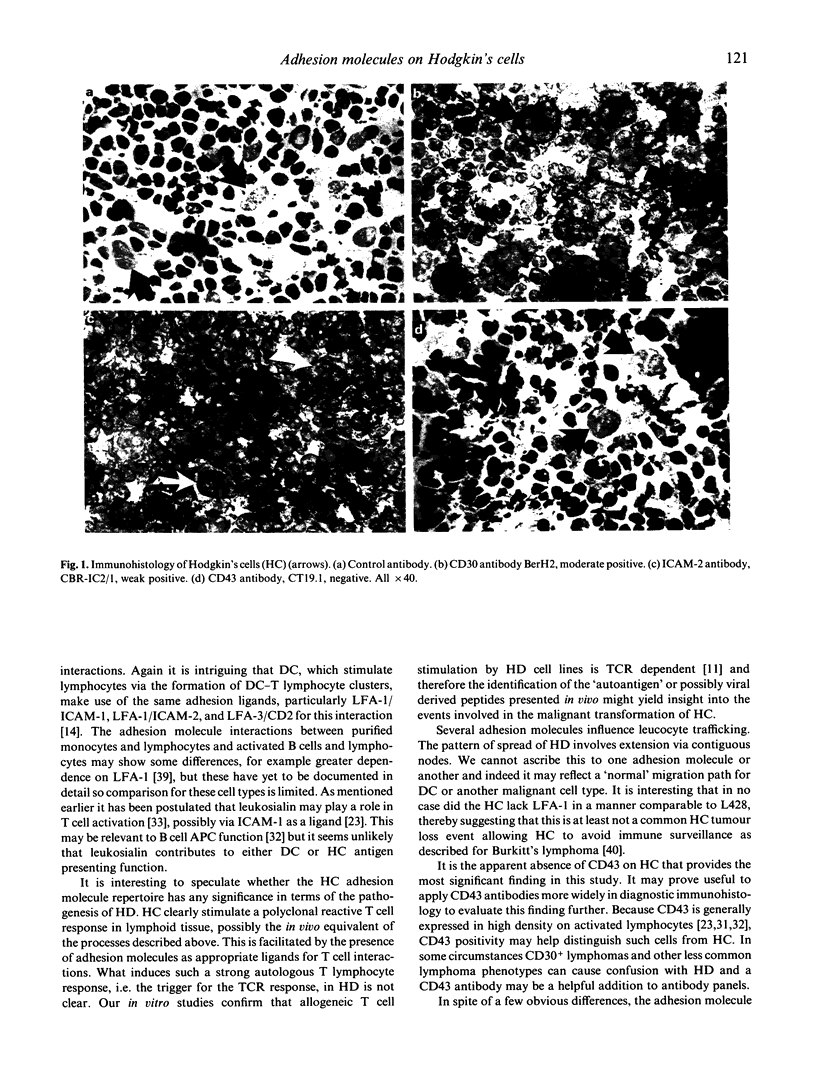


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujdoso R., Hopkins J., Dutia B. M., Young P., McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med. 1989 Oct 1;170(4):1285–1301. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey T. T., Olson S. J., Cousar J. B., Collins R. D. Immunophenotypes of Reed-Sternberg cells: a study of 19 cases of Hodgkin's disease in plastic-embedded sections. Blood. 1989 Dec;74(8):2624–2628. [PubMed] [Google Scholar]
- Clayberger C., Wright A., Medeiros L. J., Koller T. D., Link M. P., Smith S. D., Warnke R. A., Krensky A. M. Absence of cell surface LFA-1 as a mechanism of escape from immunosurveillance. Lancet. 1987 Sep 5;2(8558):533–536. doi: 10.1016/s0140-6736(87)92924-2. [DOI] [PubMed] [Google Scholar]
- Colby T. V., Hoppe R. T., Warnke R. A. Hodgkin's disease: a clinicopathologic study of 659 cases. Cancer. 1982 May 1;49(9):1848–1858. doi: 10.1002/1097-0142(19820501)49:9<1848::aid-cncr2820490918>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Davis A. L., McKenzie J. L., Hart D. N. HLA-DR-positive leucocyte subpopulations in human skin include dendritic cells, macrophages, and CD7-negative T cells. Immunology. 1988 Dec;65(4):573–581. [PMC free article] [PubMed] [Google Scholar]
- Dorreen M. S., Habeshaw J. A., Stansfeld A. G., Wrigley P. F., Lister T. A. Characteristics of Sternberg-Reed, and related cells in Hodgkin's disease: an immunohistological study. Br J Cancer. 1984 Apr;49(4):465–476. doi: 10.1038/bjc.1984.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F., Sauder D. N., Scala G., Diehl V. Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocyte cultures. J Immunol. 1983 Jun;130(6):2666–2670. [PubMed] [Google Scholar]
- Fisher R. I., Cossman J., Diehl V., Volkman D. J. Antigen presentation by Hodgkin's disease cells. J Immunol. 1985 Nov;135(5):3568–3571. [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Major histocompatibility complex antigens in rat kidney, ureter, and bladder. Localization with monoclonal antibodies and demonstration of Ia-positive dendritic cells. Transplantation. 1981 May;31(5):318–325. doi: 10.1097/00007890-198105010-00003. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Hock B. D., Hart D. N. Cellular protein profiles of the Hodgkin's disease cell lines L428, KM-H2 and HDLM-2: a comparative study. Leuk Res. 1992;16(3):253–263. doi: 10.1016/0145-2126(92)90063-d. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Muramoto L., Said J. Expression of T-cell antigens on Reed-Sternberg cells in a subset of patients with nodular sclerosing and mixed cellularity Hodgkin's disease. Am J Pathol. 1988 Feb;130(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E. Possible origin of the Reed-Sternberg cell from an interdigitating reticulum cell. Cancer Treat Rep. 1982 Apr;66(4):601–608. [PubMed] [Google Scholar]
- Kennedy I. C., Hart D. N., Colls B. M., Nimmo J. C., Willis D. A., Angus H. B. Nodular sclerosing, mixed cellularity and lymphocyte-depleted variants of Hodgkin's disease are probable dendritic cell malignancies. Clin Exp Immunol. 1989 Jun;76(3):324–331. [PMC free article] [PubMed] [Google Scholar]
- Lukes R. J., Butler J. J. The pathology and nomenclature of Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1063–1083. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Shaw S. The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition. Immunol Today. 1989 Dec;10(12):417–422. doi: 10.1016/0167-5699(89)90039-X. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Witmer-Pack M. D., Agger R., Crowley M. T., Lawless D., Steinman R. M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990 May 1;171(5):1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. K., Rosenstein Y. J., Remold-O'Donnell E., Bierer B. E., Rosen F. S., Burakoff S. J. Enhancement of T-cell activation by the CD43 molecule whose expression is defective in Wiskott-Aldrich syndrome. Nature. 1991 Apr 25;350(6320):706–709. doi: 10.1038/350706a0. [DOI] [PubMed] [Google Scholar]
- Payne S. V., Wright D. H., Jones K. J., Judd M. A. Macrophage origin of Reed-Sternberg cells: an immunohistochemical study. J Clin Pathol. 1982 Feb;35(2):159–166. doi: 10.1136/jcp.35.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett T. C., McKenzie J. L., Hart D. N. Adhesion molecules on human tonsil dendritic cells. Transplantation. 1992 Feb;53(2):483–490. doi: 10.1097/00007890-199202010-00041. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Zimmerman C., Kenney D., Rosen F. S. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987 Jul;70(1):104–109. [PubMed] [Google Scholar]
- Rosenstein Y., Park J. K., Hahn W. C., Rosen F. S., Bierer B. E., Burakoff S. J. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991 Nov 21;354(6350):233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- Schmid C., Pan L., Diss T., Isaacson P. G. Expression of B-cell antigens by Hodgkin's and Reed-Sternberg cells. Am J Pathol. 1991 Oct;139(4):701–707. [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Siraganian R., Wahl L., Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989 Oct 15;143(8):2457–2463. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Springer T. A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989 May 4;339(6219):61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Strauchen J. A. Immunopathology of Hodgkin's disease. Pathol Immunopathol Res. 1986;5(3-5):253–264. doi: 10.1159/000157019. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Penta A. C., Levine H. B., Freedman A. S. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J Immunol. 1990 Jan 15;144(2):532–540. [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Hu E., Warnke R. A., Sklar J. Immunoglobulin gene rearrangements in Hodgkin's disease. Hum Pathol. 1986 Oct;17(10):1009–1014. doi: 10.1016/s0046-8177(86)80084-3. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Springer T. A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992 Jan 1;175(1):185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]



