Abstract
Cytokines are important mediators involved in the development of effector cells and in the regulation of immune responses. The gene expression of these mediators in T cell subset has yet to be fully elucidated. Using sensitive reverse transcription-polymerase chain reaction (RT-PCR), the kinetics of cytokine gene expression in human CD4+ and CD8+ T cells were examined. CD4+ T cells were more readily activated by phorbol myristate acetate (PMA) and phytohaemagglutinin (PHA) than CD8+ T cells in terms of the IL-2 receptor (IL-2R) mRNA expression. Quantitative differences in cytokine gene expression between CD4+ and CD8+ T cells were confirmed and higher levels of cytokine mRNAs were induced in CD4+ than in CD8+ T cells. Early induction of IL-2 mRNA was observed in both T cell subsets. The demonstration of different kinetics of cytokine gene expression illustrates one of the examples of the complexity of immunoregulation. The differential response of cytokine gene expression in different T cell subsets should be taken into consideration when clinical studies in cytokine production by peripheral blood mononuclear cells are interpreted.
Full text
PDF

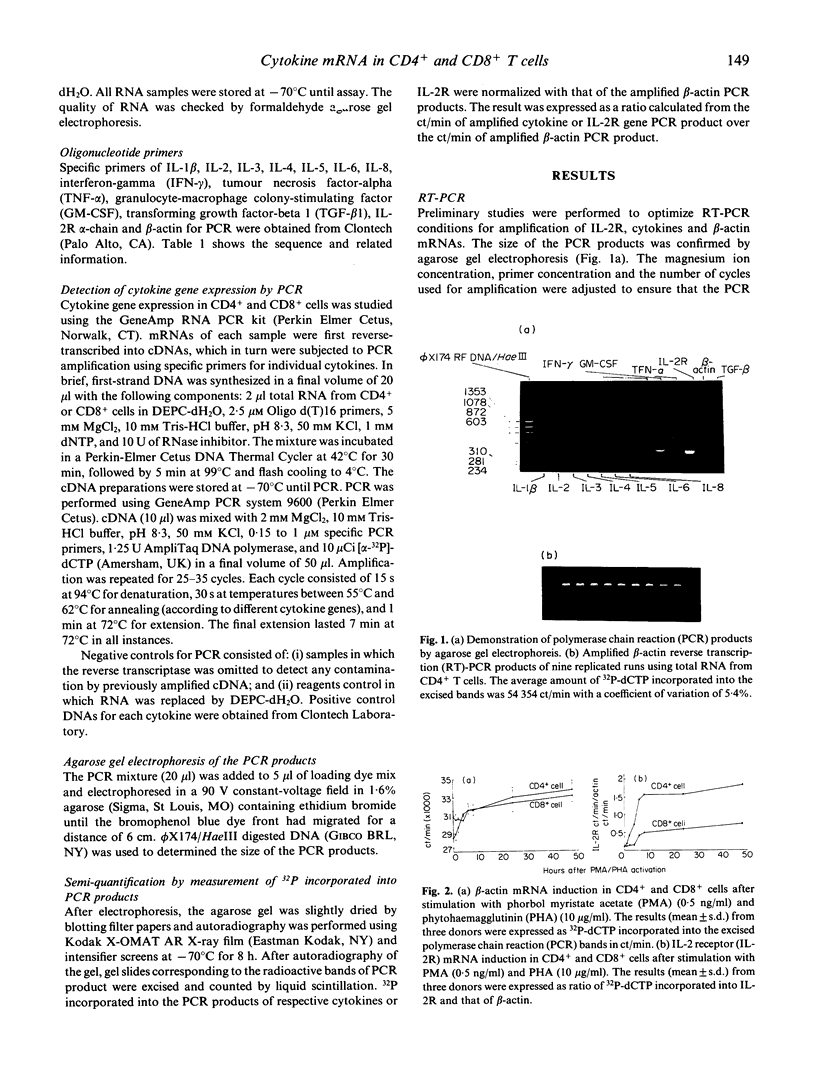
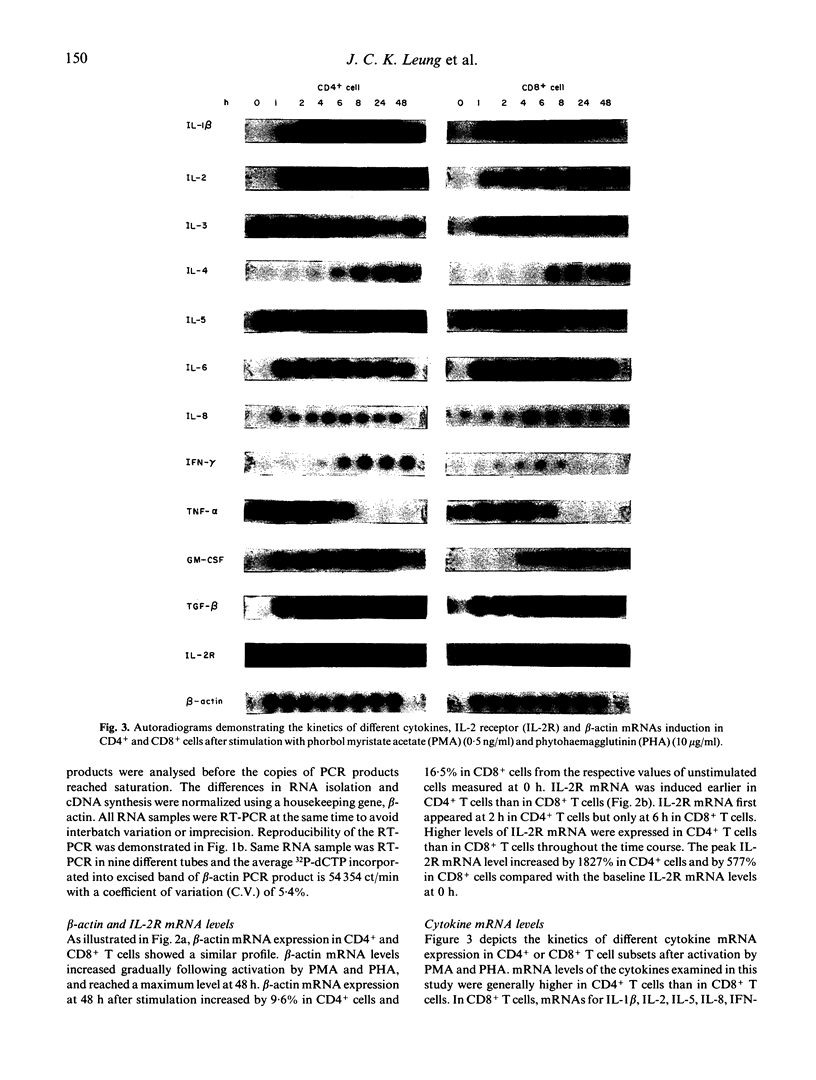
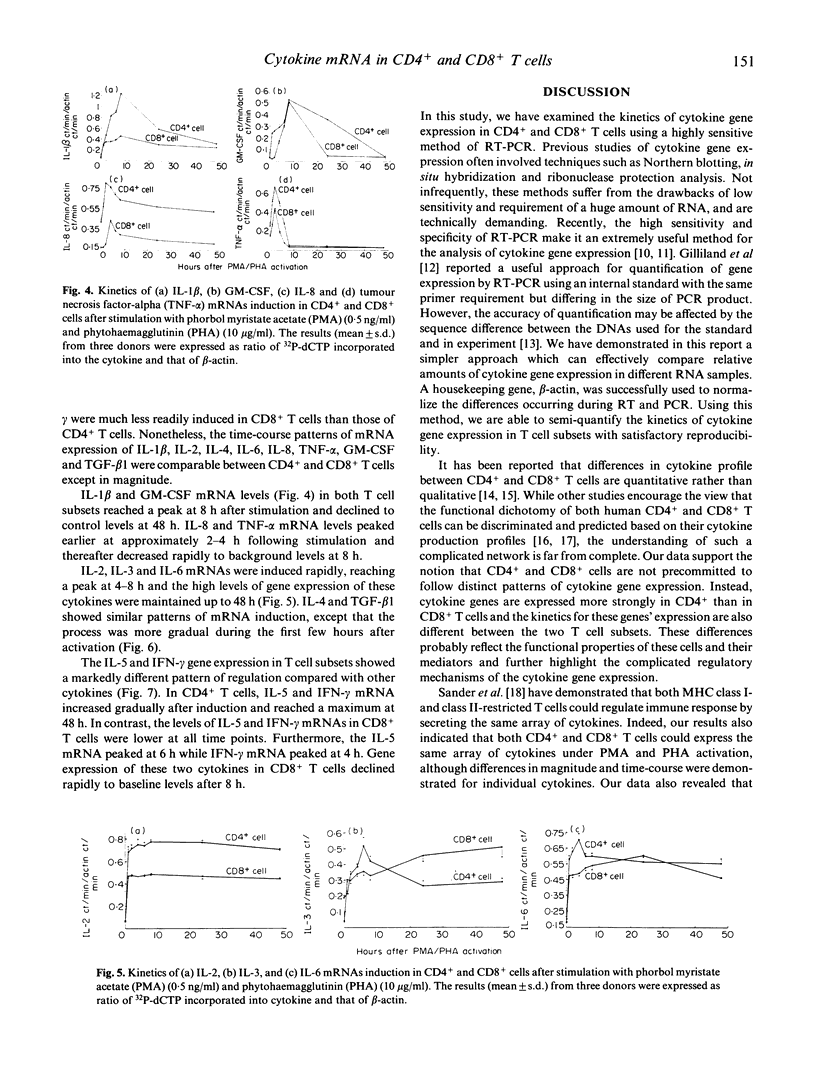


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner C. A., Tam A. W., Nelson P. A., Engleman E. G., Suzuki N., Fry K. E., Larrick J. W. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989 Nov-Dec;7(10):1096–1103. [PubMed] [Google Scholar]
- Carter B. Z., Malter J. S. Regulation of mRNA stability and its relevance to disease. Lab Invest. 1991 Dec;65(6):610–621. [PubMed] [Google Scholar]
- Coles M., Rose M., Yacoub M. Appearance of cells bearing the interleukin-2 receptor in peripheral blood of cardiac transplant patients and their correlation with rejection episodes. Transplant Proc. 1987 Feb;19(1 Pt 3):2546–2547. [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Gauchat J. F., Walker C., De Weck A. L., Stadler B. M. Stimulation-dependent lymphokine mRNA levels in human mononuclear cells. Eur J Immunol. 1988 Sep;18(9):1441–1446. doi: 10.1002/eji.1830180921. [DOI] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L. Secretion of interleukin 2, macrophage-activating factor, interferon, and colony-stimulating factor by alloreactive T lymphocyte clones. J Immunol. 1984 Jun;132(6):2924–2931. [PubMed] [Google Scholar]
- Kelso A., Troutt A. B., Maraskovsky E., Gough N. M., Morris L., Pech M. H., Thomson J. A. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol Rev. 1991 Oct;123:85–114. doi: 10.1111/j.1600-065x.1991.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Kim T., Kanayama Y., Negoro N., Okamura M., Takeda T., Inoue T. Serum levels of interferons in patients with systemic lupus erythematosus. Clin Exp Immunol. 1987 Dec;70(3):562–569. [PMC free article] [PubMed] [Google Scholar]
- Lai K. N., Leung J. C., Lai F. M., Tam J. S. T-lymphocyte activation in IgA nephropathy: serum-soluble interleukin 2 receptor level, interleukin 2 production, and interleukin 2 receptor expression by cultured lymphocytes. J Clin Immunol. 1989 Nov;9(6):485–492. doi: 10.1007/BF00918018. [DOI] [PubMed] [Google Scholar]
- Lai K. N., Leung J. C., Li P. K., Lui S. F. Cytokine production by peripheral blood mononuclear cells in IgA nephropathy. Clin Exp Immunol. 1991 Aug;85(2):240–245. doi: 10.1111/j.1365-2249.1991.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Werb Z. mRNA phenotyping for studying gene expression in small numbers of cells: platelet-derived growth factor and other growth factors in wound-derived macrophages. Am J Respir Cell Mol Biol. 1990 Jan;2(1):3–10. doi: 10.1165/ajrcmb/2.1.3. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Strath M., Warren D. J., O'Garra A., Kirkwood T. B. The production of lymphokines by primary alloreactive T-cell clones: a co-ordinate analysis of 233 clones in seven lymphokine assays. Immunology. 1985 Dec;56(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Semenzato G., Bambara L. M., Biasi D., Frigo A., Vinante F., Zuppini B., Trentin L., Feruglio C., Chilosi M., Pizzolo G. Increased serum levels of soluble interleukin-2 receptor in patients with systemic lupus erythematosus and rheumatoid arthritis. J Clin Immunol. 1988 Nov;8(6):447–452. doi: 10.1007/BF00916949. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Lee T. H. Expression of interleukin-5 and granulocyte-macrophage colony-stimulating factor in human peripheral blood mononuclear cells after activation with phorbol myristate acetate. Immunology. 1992 Jan;75(1):196–201. [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Jabara H. H., DeKruyff R. H., Abbas A. K., Abrams J. S., Geha R. S. Functional heterogeneity among human inducer T cell clones. J Immunol. 1988 Jun 15;140(12):4211–4216. [PubMed] [Google Scholar]
- Vilcek J., Henriksen-Destefano D., Siegel D., Klion A., Robb R. J., Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985 Sep;135(3):1851–1856. [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejstål R., Norkrans G., Weiland O., Schvarcz R., Fuchs D., Wachter H., Fryden A., Glaumann H. Lymphocyte subsets and beta 2-microglobulin expression in chronic hepatitis C/non-A, non-B. Effects of interferon-alpha treatment. Clin Exp Immunol. 1992 Mar;87(3):340–345. doi: 10.1111/j.1365-2249.1992.tb02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh S. L., Gough N. M., Finlay-Jones J. J., McDonald P. J. Detection of cytokine mRNA in astrocyte cultures using the polymerase chain reaction. Lymphokine Res. 1990 Summer;9(2):177–185. [PubMed] [Google Scholar]



