Abstract
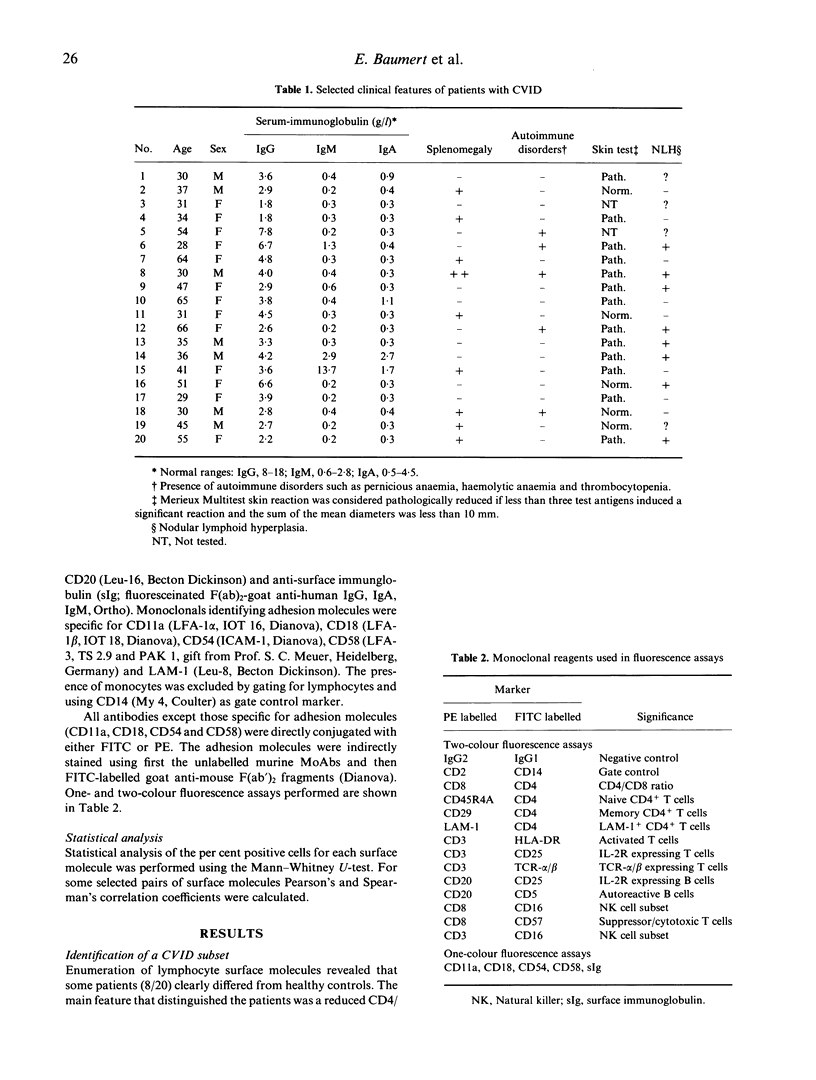
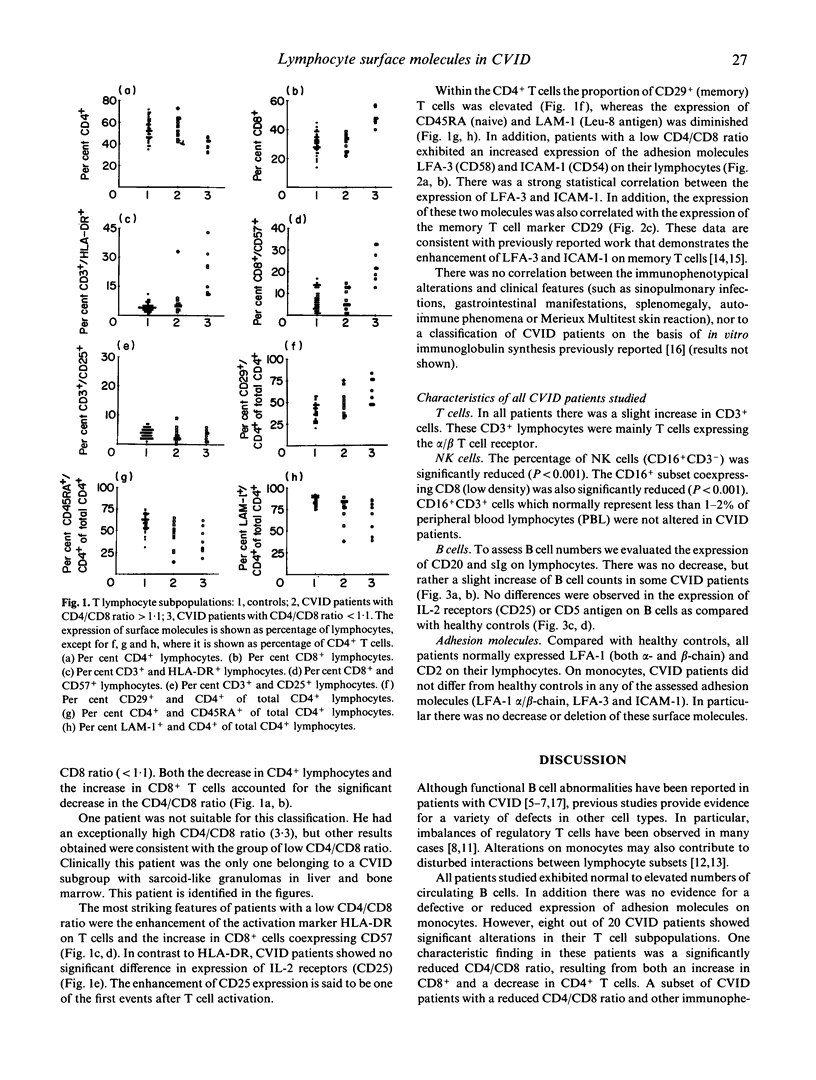
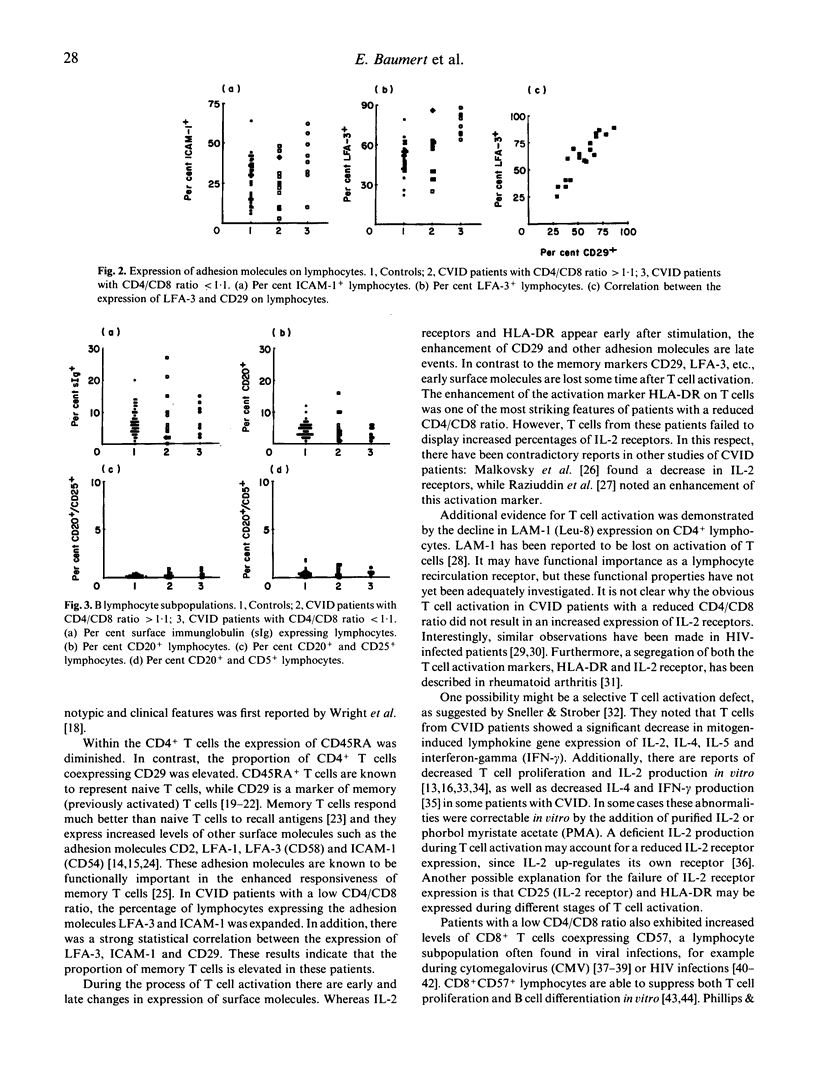
We investigated the expression of surface molecules on lymphocytes from 20 patients with CVID and 40 healthy subjects. Lymphocytes were analysed by dual colour flow cytometry. We identified a subset of patients (8 of 20) characterized by low CD4/CD8 ratio (less than 1.1), expansion of T cells co-expressing the activation marker HLA-DR and significant increase in CD8+ T cells co-expressing CD57. Expression of the adhesion molecules LFA-3 (CD58) and ICAM-1 (CD54) was significantly increased in this subgroup. In addition, within the CD4+ T cells the percentage of CD29+ (memory) cells was increased, while the CD45RA and LAM-1 (Leu-8) antigens were depressed. These results indicate that in a subgroup of CVID patients T cells are activated in vivo and the CD57+CD8+ lymphocyte subpopulation, supposed to comprise functional suppressor T cells, is expanded. We suggest a chronic viral infection in these patients, but it is not clear whether this is primary or secondary to the underlying defect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Bogner J. R., Matuschke A., Heinrich B., Schreiber M. A., Nerl C., Goebel F. D. Expansion of activated T lymphocytes (CD3 + HLA/DR +) detectable in early stages of HIV-1 infection. Klin Wochenschr. 1990 Apr 17;68(8):393–396. doi: 10.1007/BF01648577. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Byrne J. A., Butler J. L., Cooper M. D. Differential activation requirements for virgin and memory T cells. J Immunol. 1988 Nov 15;141(10):3249–3257. [PubMed] [Google Scholar]
- Clement L. T., Grossi C. E., Gartland G. L. Morphologic and phenotypic features of the subpopulation of Leu-2+ cells that suppresses B cell differentiation. J Immunol. 1984 Nov;133(5):2461–2468. [PubMed] [Google Scholar]
- Cunningham-Rundles C., Siegal F. P., Cunningham-Rundles S., Lieberman P. Incidence of cancer in 98 patients with common varied immunodeficiency. J Clin Immunol. 1987 Jul;7(4):294–299. doi: 10.1007/BF00915550. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Cunningham-Rundles C., Siegal F. P., Gupta S., Smithwick E. M., Kosloff C., Good R. A. Defective cellular immune response in vitro in common variable immunodeficiency. J Clin Immunol. 1981 Jan;1(1):65–72. doi: 10.1007/BF00915478. [DOI] [PubMed] [Google Scholar]
- Döcke W. D., Simon H. U., Fietze E., Prösch S., Diener C., Reinke P., Stein H., Volk H. D. Cytomegalovirus infection and common variable immunodeficiency. Lancet. 1991 Dec 21;338(8782-8783):1597–1597. doi: 10.1016/0140-6736(91)92422-x. [DOI] [PubMed] [Google Scholar]
- Farrant J., Bryant A. E., Lever A. M., Edwards A. J., Knight S. C., Webster A. D. Defective low-density cells of dendritic morphology from the blood of patients with common variable hypogammaglobulinaemia: low immunoglobulin production on stimulation of normal B cells. Clin Exp Immunol. 1985 Jul;61(1):189–194. [PMC free article] [PubMed] [Google Scholar]
- Fiedler W., Sykora K. W., Welte K., Kolitz J. E., Cunningham-Rundles C., Holloway K., Miller G. A., Souza L., Mertelsmann R. T-cell activation defect in common variable immunodeficiency: restoration by phorbol myristate acetate (PMA) or allogeneic macrophages. Clin Immunol Immunopathol. 1987 Aug;44(2):206–218. doi: 10.1016/0090-1229(87)90066-3. [DOI] [PubMed] [Google Scholar]
- Friedman R., Ackerman M., Mallory G., Weng T. R., Fireman P. Hypogammaglobulinemia with sarcoidlike granulomas. Am J Dis Child. 1983 Aug;137(8):774–776. doi: 10.1001/archpedi.1983.02140340054015. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Nishanian P. G., Schmid I., Hultin L. E., Cheng H. L., Detels R. Selective alterations in immunoregulatory lymphocyte subsets in early HIV (human T-lymphotropic virus type III/lymphadenopathy-associated virus) infection. J Clin Immunol. 1987 Mar;7(2):140–150. doi: 10.1007/BF00916008. [DOI] [PubMed] [Google Scholar]
- Gratama J. W., Kardol M., Naipal A. M., Slats J., Den Ouden A., Stijnen T., D'Amaro J., The T. H., Bruning J. W. The influence of cytomegalovirus carrier status on lymphocyte subsets and natural immunity. Clin Exp Immunol. 1987 Jul;69(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Greenberger P. A., Walker C. L., Fitzsimons T. E., Roberts M. Hypogammaglobulinemia associated with cytomegalovirus pneumonia. J Infect Dis. 1991 Mar;163(3):631–633. doi: 10.1093/infdis/163.3.631. [DOI] [PubMed] [Google Scholar]
- Gupta S. Abnormality of Leu 2+7+ cells in acquired immune deficiency syndrome (AIDS), AIDS-related complex, and asymptomatic homosexuals. J Clin Immunol. 1986 Nov;6(6):502–509. doi: 10.1007/BF00915256. [DOI] [PubMed] [Google Scholar]
- Gupta S. Study of activated T cells in man. II. Interleukin 2 receptor and transferrin receptor expression on T cells and production of interleukin 2 in patients with acquired immune deficiency syndrome (AIDS) and AIDS-related complex. Clin Immunol Immunopathol. 1986 Jan;38(1):93–100. doi: 10.1016/0090-1229(86)90126-1. [DOI] [PubMed] [Google Scholar]
- Howe H. S., So A. K., Farrant J., Webster A. D. Common variable immunodeficiency is associated with polymorphic markers in the human major histocompatibility complex. Clin Exp Immunol. 1991 Mar;83(3):387–390. doi: 10.1111/j.1365-2249.1991.tb05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanof M. E., James S. P. Leu-8 antigen expression is diminished during cell activation but does not correlate with effector function of activated T lymphocytes. J Immunol. 1988 Jun 1;140(11):3701–3706. [PubMed] [Google Scholar]
- Kinlen L. J., Webster A. D., Bird A. G., Haile R., Peto J., Soothill J. F., Thompson R. A. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985 Feb 2;1(8423):263–266. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- Kruger G., Welte K., Ciobanu N., Cunningham-Rundles C., Ralph P., Venuta S., Feldman S., Koziner B., Wang C. Y., Moore M. A. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984 Jul;4(4):295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- Landay A., Poon M. C., Clement L. T., Grossi C. E. A lymphoproliferative disorder of granular lymphocytes with a novel phenotype and suppressor function. J Clin Immunol. 1984 Jul;4(4):326–334. doi: 10.1007/BF00915301. [DOI] [PubMed] [Google Scholar]
- Legendre C. M., Guttmann R. D., Hou S. K., Jean R. Two-color immunofluorescence and flow cytometry analysis of lymphocytes in long-term renal allotransplant recipients: identification of a major Leu-7+/Leu-3+ subpopulation. J Immunol. 1985 Aug;135(2):1061–1066. [PubMed] [Google Scholar]
- Mackay C. R. T-cell memory: the connection between function, phenotype and migration pathways. Immunol Today. 1991 Jun;12(6):189–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Jíra M., Gao L., Loveland B., Malkovska V., Dalgleish A. G., Webster A. D. Reduced expression of interleukin-2 receptors in hypogammaglobulinaemia: a possible cause of higher cancer incidence. Lancet. 1986 Jun 21;1(8495):1442–1443. doi: 10.1016/s0140-6736(86)91586-2. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Oliveira D. B., Winearls C. G., Cohen J., Ind P. W., Williams G. Severe immunosuppression in a renal transplant recipient with HTLV-III antibodies. Transplantation. 1986 Feb;41(2):260–262. doi: 10.1097/00007890-198602000-00024. [DOI] [PubMed] [Google Scholar]
- Pastorelli G., Roncarolo M. G., Touraine J. L., Peronne G., Tovo P. A., de Vries J. E. Peripheral blood lymphocytes of patients with common variable immunodeficiency (CVI) produce reduced levels of interleukin-4, interleukin-2 and interferon-gamma, but proliferate normally upon activation by mitogens. Clin Exp Immunol. 1989 Dec;78(3):334–340. [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Lectin-dependent and anti-CD3 induced cytotoxicity are preferentially mediated by peripheral blood cytotoxic T lymphocytes expressing Leu-7 antigen. J Immunol. 1986 Mar 1;136(5):1579–1585. [PubMed] [Google Scholar]
- Plaeger-Marshall S., Spina C. A., Giorgi J. V., Mitsuyasu R., Wolfe P., Gottlieb M., Beall G. Alterations in cytotoxic and phenotypic subsets of natural killer cells in acquired immune deficiency syndrome (AIDS). J Clin Immunol. 1987 Jan;7(1):16–23. doi: 10.1007/BF00915420. [DOI] [PubMed] [Google Scholar]
- Provisor A. J., Iacuone J. J., Chilcote R. R., Neiburger R. G., Crussi F. G. Acquired agammaglobulinemia after a life-threatening illness with clinical and laboratory features of infectious mononucleosis in three related male children. N Engl J Med. 1975 Jul 10;293(2):62–65. doi: 10.1056/NEJM197507102930202. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., DeFlorio D., Jr, Hutt L. M., Bhawan J., Yang J. P., Otto R., Edwards W. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N Engl J Med. 1977 Nov 17;297(20):1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- Raziuddin S., Elawad M. E., Benjamin B. T-cell abnormalities in antibody deficiency syndromes. Scand J Immunol. 1989 Oct;30(4):419–424. doi: 10.1111/j.1365-3083.1989.tb02445.x. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Cooper M. D., Schlossman S. F., Rosen F. S. Abnormalities of T cell maturation and regulation in human beings with immunodeficiency disorders. J Clin Invest. 1981 Sep;68(3):699–705. doi: 10.1172/JCI110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Geha R., Wohl M. E., Morimoto C., Rosen F. S., Schlossman S. F. Immunodeficiency associated with loss of T4+ inducer T-cell function. N Engl J Med. 1981 Apr 2;304(14):811–816. doi: 10.1056/NEJM198104023041403. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. A., Bankhurst A. D., Williams R. C., Jr Characterization of the suppressor activity in lymphocytes from patients with common variable hypogammaglobulinemia: evidence for an associated primary B-cell defect. Clin Immunol Immunopathol. 1983 Oct;29(1):35–50. doi: 10.1016/0090-1229(83)90005-3. [DOI] [PubMed] [Google Scholar]
- Rüthlein J., James S. P., Strober W. Role of CD2 in activation and cytotoxic function of CD8/Leu-7-positive T cells. J Immunol. 1988 Dec 1;141(11):3791–3797. [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Saxon A., Giorgi J. V., Sherr E. H., Kagan J. M. Failure of B cells in common variable immunodeficiency to transit from proliferation to differentiation is associated with altered B cell surface-molecule display. J Allergy Clin Immunol. 1989 Jul;84(1):44–55. doi: 10.1016/0091-6749(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Choi Y. S., Shou L., Good R. A. Modulatory effects on immunoglobulin synthesis and secretion by lymphocytes from immunodeficient patients. J Clin Invest. 1977 Jun;59(6):1176–1187. doi: 10.1172/JCI108742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller M. C., Strober W. Abnormalities of lymphokine gene expression in patients with common variable immunodeficiency. J Immunol. 1990 May 15;144(10):3762–3769. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tosato G., Magrath I., Koski I., Dooley N., Blaese M. Activation of suppressor T cells during Epstein-Barr-virus-induced infectious mononucleosis. N Engl J Med. 1979 Nov 22;301(21):1133–1137. doi: 10.1056/NEJM197911223012101. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- Wallace D. L., Beverley P. C. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990 Mar;69(3):460–467. [PMC free article] [PubMed] [Google Scholar]
- Wright J. J., Wagner D. K., Blaese R. M., Hagengruber C., Waldmann T. A., Fleisher T. A. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990 Nov 15;76(10):2046–2051. [PubMed] [Google Scholar]
- de la Concha E. G., Oldham G., Webster A. D., Asherson G. L., Platts-Mills T. A. Quantitative measurements of T- and B-cell function in "variable" primary hypogammaglobulinaemia: evidence for a consistent B-cell defect. Clin Exp Immunol. 1977 Feb;27(2):208–215. [PMC free article] [PubMed] [Google Scholar]


