Abstract
Mitoxantrone was used as an immunosuppressive probe to elucidate a means for the control of experimental allergic encephalomyelitis (EAE) induced in Biozzi AB/H mice following injection of spinal cord homogenate emulsified in Freund's adjuvant. A single i.p. injection of 2.5 mg/kg of mitoxantrone, 1-2 days before the anticipated onset of EAE, failed to prevent the majority of animals from developing clinical disease, whereas when the compound was injected directly into the central nervous system (CNS), at this time point, significantly increased therapeutic benefit was evident, with most animals failing to develop clinical EAE. Although the clinical use of intrathecal mitoxantrone is strongly contraindicated, these data suggest that increased therapeutic benefit may be achieved in immune-mediated disease of the CNS by targeting immunosuppressive doses of suitable agents, on lymphocyte activation within the CNS. In addition, direct administration of immunosuppressive doses into the CNS may reduce potentially unwanted (side) effects in the periphery.
Full text
PDF

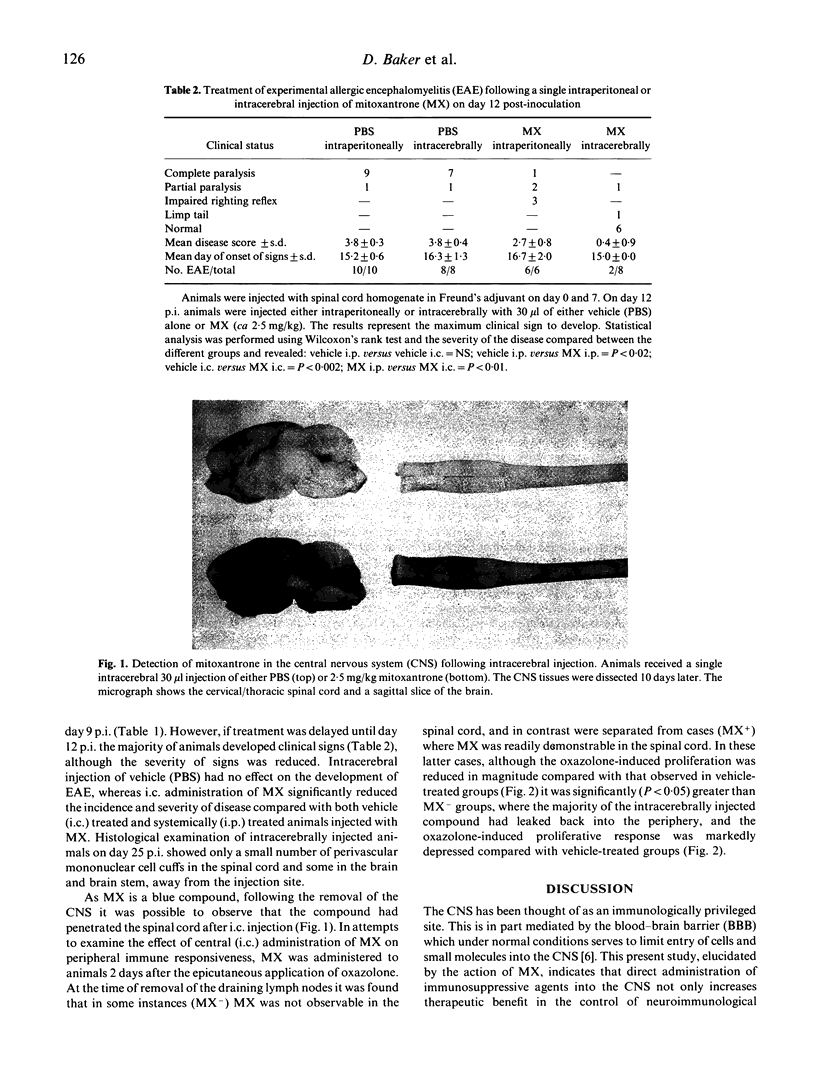


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D., Davison A. N. Mechanisms of immune-mediated demyelinating disease of the central nervous system. Neurochem Res. 1991 Sep;16(9):1067–1072. doi: 10.1007/BF00965852. [DOI] [PubMed] [Google Scholar]
- Baker D., O'Neill J. K., Amor S., Khamashta M. A., Turk J. L. Inhibition of chronic relapsing experimental allergic encephalomyelitis in the mouse by the alkyl-lysophospholipid ET-18-OCH3. Int J Immunopharmacol. 1991;13(4):385–392. doi: 10.1016/0192-0561(91)90008-u. [DOI] [PubMed] [Google Scholar]
- Baker D., O'Neill J. K., Gschmeissner S. E., Wilcox C. E., Butter C., Turk J. L. Induction of chronic relapsing experimental allergic encephalomyelitis in Biozzi mice. J Neuroimmunol. 1990 Aug;28(3):261–270. doi: 10.1016/0165-5728(90)90019-j. [DOI] [PubMed] [Google Scholar]
- Benvenuto R., Paroli M., Buttinelli C., Franco A., Barnaba V., Fieschi C., Balsano F. Tumour necrosis factor-alpha synthesis by cerebrospinal-fluid-derived T cell clones from patients with multiple sclerosis. Clin Exp Immunol. 1991 Apr;84(1):97–102. [PMC free article] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butter C., Baker D., O'Neill J. K., Turk J. L. Mononuclear cell trafficking and plasma protein extravasation into the CNS during chronic relapsing experimental allergic encephalomyelitis in Biozzi AB/H mice. J Neurol Sci. 1991 Jul;104(1):9–12. doi: 10.1016/0022-510x(91)90209-p. [DOI] [PubMed] [Google Scholar]
- Butter C., O'Neill J. K., Baker D., Gschmeissner S. E., Turk J. L. An immunoelectron microscopical study of the expression of class II major histocompatibility complex during chronic relapsing experimental allergic encephalomyelitis in Biozzi AB/H mice. J Neuroimmunol. 1991 Jul;33(1):37–42. doi: 10.1016/0165-5728(91)90032-3. [DOI] [PubMed] [Google Scholar]
- Calder V., Owen S., Watson C., Feldmann M., Davison A. MS: a localized immune disease of the central nervous system. Immunol Today. 1989 Mar;10(3):99–103. doi: 10.1016/0167-5699(89)90235-1. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Cannella B., Brosnan C. F., Raine C. S. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990 Aug;63(2):162–170. [PubMed] [Google Scholar]
- Durr F. E., Wallace R. E., Citarella R. V. Molecular and biochemical pharmacology of mitoxantrone. Cancer Treat Rev. 1983 Dec;10 (Suppl B):3–11. doi: 10.1016/0305-7372(83)90016-6. [DOI] [PubMed] [Google Scholar]
- Green R. M., Stewart D. J., Hugenholtz H., Richard M. T., Thibault M., Montpetit V. Human central nervous system and plasma pharmacology of mitoxantrone. J Neurooncol. 1988;6(1):75–83. doi: 10.1007/BF00163544. [DOI] [PubMed] [Google Scholar]
- Hall C., Dougherty W. J., Lebish I. J., Brock P. G., Man A. Warning against use of intrathecal mitoxantrone. Lancet. 1989 Apr 1;1(8640):734–734. doi: 10.1016/s0140-6736(89)92256-3. [DOI] [PubMed] [Google Scholar]
- Kermode A. G., Thompson A. J., Tofts P., MacManus D. G., Kendall B. E., Kingsley D. P., Moseley I. F., Rudge P., McDonald W. I. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990 Oct;113(Pt 5):1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Lakhani A. K., Zuiable A. G., Pollard C. M., Milne A., Treleaven J., Powles R. L. Paraplegia after intrathecal mitozantrone. Lancet. 1986 Dec 13;2(8520):1393–1393. doi: 10.1016/s0140-6736(86)92030-1. [DOI] [PubMed] [Google Scholar]
- Levine S., Saltzman A. Regional suppression, therapy after onset and prevention of relapses in experimental allergic encephalomyelitis by mitoxantrone. J Neuroimmunol. 1986 Dec;13(2):175–181. doi: 10.1016/0165-5728(86)90063-9. [DOI] [PubMed] [Google Scholar]
- Lu K., Savaraj N., Loo T. L. Pharmacological disposition of 1,4-dihydroxy-5-8-bis[[2 [(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione dihydrochloride in the dog. Cancer Chemother Pharmacol. 1984;13(1):63–66. doi: 10.1007/BF00401450. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., McFarlin D. E., Raine C. S. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984 May 24;309(5966):356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- O'Neill J. K., Baker D., Davison A. N., Maggon K. K., Jaffee B. D., Turk J. L. Therapy of chronic relapsing experimental allergic encephalomyelitis and the role of the blood-brain barrier: elucidation by the action of Brequinar sodium. J Neuroimmunol. 1992 May;38(1-2):53–62. doi: 10.1016/0165-5728(92)90090-8. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Lee S. C., Scheinberg L. C., Duijvestin A. M., Cross A. H. Adhesion molecules on endothelial cells in the central nervous system: an emerging area in the neuroimmunology of multiple sclerosis. Clin Immunol Immunopathol. 1990 Nov;57(2):173–187. doi: 10.1016/0090-1229(90)90032-l. [DOI] [PubMed] [Google Scholar]
- Roboz J., Paciucci P. A., Silides D., Greaves J., Holland J. F. Detection and quantification of mitoxantrone in human organs. A case report. Cancer Chemother Pharmacol. 1984;13(1):67–68. doi: 10.1007/BF00401451. [DOI] [PubMed] [Google Scholar]
- Stern Y., Tetrud J. W., Martin W. R., Kutner S. J., Langston J. W. Cognitive change following MPTP exposure. Neurology. 1990 Feb;40(2):261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- Stern Y., Tetrud J. W., Martin W. R., Kutner S. J., Langston J. W. Cognitive change following MPTP exposure. Neurology. 1990 Feb;40(2):261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Green R. M., Mikhael N. Z., Montpetit V., Thibault M., Maroun J. A. Human autopsy tissue concentrations of mitoxantrone. Cancer Treat Rep. 1986 Nov;70(11):1255–1261. [PubMed] [Google Scholar]
- Sun J. B., Olsson T., Wang W. Z., Xiao B. G., Kostulas V., Fredrikson S., Ekre H. P., Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991 Jun;21(6):1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- Unger C., Eibl H., von Heyden H. W., Krisch B., Nagel G. A. Blut-Hirnschranke und Penetration von Zytostatika. Klin Wochenschr. 1985 Jun 18;63(12):565–571. doi: 10.1007/BF01733202. [DOI] [PubMed] [Google Scholar]
- Voorthuis J. A., Uitdehaag B. M., De Groot C. J., Goede P. H., van der Meide P. H., Dijkstra C. D. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol. 1990 Aug;81(2):183–188. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. M., Davison A. N., Baker D., O'Neill J. K., Turk J. L. Suppression of demyelination by mitoxantrone. Int J Immunopharmacol. 1991;13(7):923–930. doi: 10.1016/0192-0561(91)90045-9. [DOI] [PubMed] [Google Scholar]



