Abstract
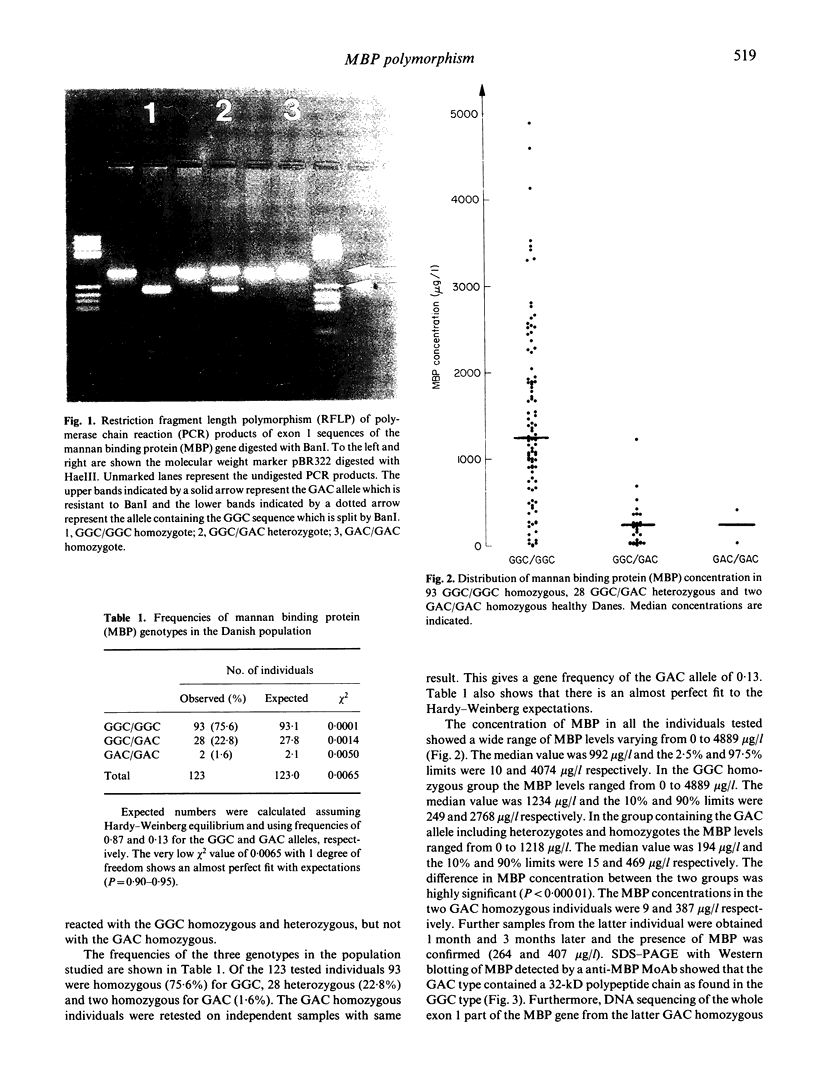
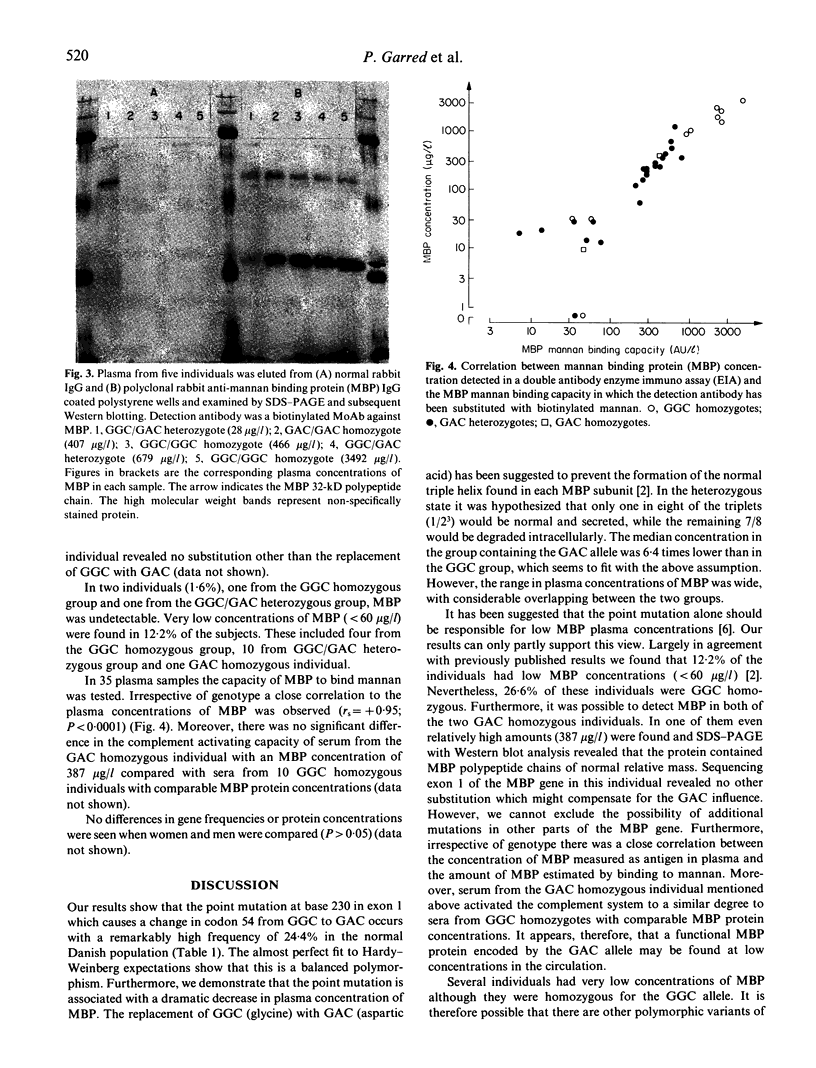
Low plasma concentration of mannan binding protein (MBP) has been shown to be the basis for a common opsonic deficiency and suggested to be caused by a single nucleotide substitution at base 230 of exon 1 in the MBP gene. This substitution causes a replacement of glycine (codon GGC) with aspartic acid (codon GAC). Of 123 healthy Danish individuals investigated by polymerase chain reaction performed on exon 1, followed by restriction fragment length polymorphism or allospecific probing, 93 were homozygous (75.6%) for GGC, 28 heterozygous (22.8%), and two homozygous for GAC (1.6%). The gene frequency of the GAC allele was found to be 0.13. DNA sequencing of the cloned exon 1 from one GAC homozygous individual revealed no other substitution. The median MBP concentration in the group containing the GAC allele was 6.4 times lower than in the GGC homozygous group (195 and 1234 micrograms/l respectively). However, the range in plasma concentrations of MBP was wide and overlapping between the groups. MBP protein was detected in both the GAC homozygotes (9 and 387 micrograms/l). Furthermore, no difference in relative mass and biological activity (mannan binding) was found when sera containing the two forms of MBP were investigated. Accordingly, it can be concluded that the GAC allele is able to produce a functional MBP protein which may be detected in serum at low concentrations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Hugo F., Tranum-Jensen J. Functions and relevance of the terminal complement sequence. Blut. 1990 Jun;60(6):309–318. doi: 10.1007/BF01737843. [DOI] [PubMed] [Google Scholar]
- Böhme J., Owerbach D., Denaro M., Lernmark A., Peterson P. A., Rask L. Human class II major histocompatibility antigen beta-chains are derived from at least three loci. Nature. 1983 Jan 6;301(5895):82–84. doi: 10.1038/301082a0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Day L. E., Herman G. A. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988 Mar 1;167(3):1034–1046. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Sannoh T., Kawasaki N., Kawasaki T., Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987 Jun 5;262(16):7451–7454. [PubMed] [Google Scholar]
- Lu J. H., Thiel S., Wiedemann H., Timpl R., Reid K. B. Binding of the pentamer/hexamer forms of mannan-binding protein to zymosan activates the proenzyme C1r2C1s2 complex, of the classical pathway of complement, without involvement of C1q. J Immunol. 1990 Mar 15;144(6):2287–2294. [PubMed] [Google Scholar]
- Morgan B. P., Walport M. J. Complement deficiency and disease. Immunol Today. 1991 Sep;12(9):301–306. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiya M., Super M., Tabona P., Levinsky R. J., Arai T., Turner M. W., Summerfield J. A. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991 Jun 29;337(8757):1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- Super M., Thiel S., Lu J., Levinsky R. J., Turner M. W. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989 Nov 25;2(8674):1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- Thiel S., Holmskov U., Hviid L., Laursen S. B., Jensenius J. C. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992 Oct;90(1):31–35. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. W. Deficiency of mannan binding protein--a new complement deficiency syndrome. Clin Exp Immunol. 1991 Oct;86 (Suppl 1):53–56. doi: 10.1111/j.1365-2249.1991.tb06208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]