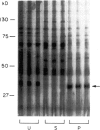
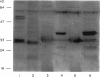
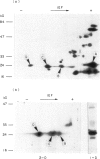
Abstract
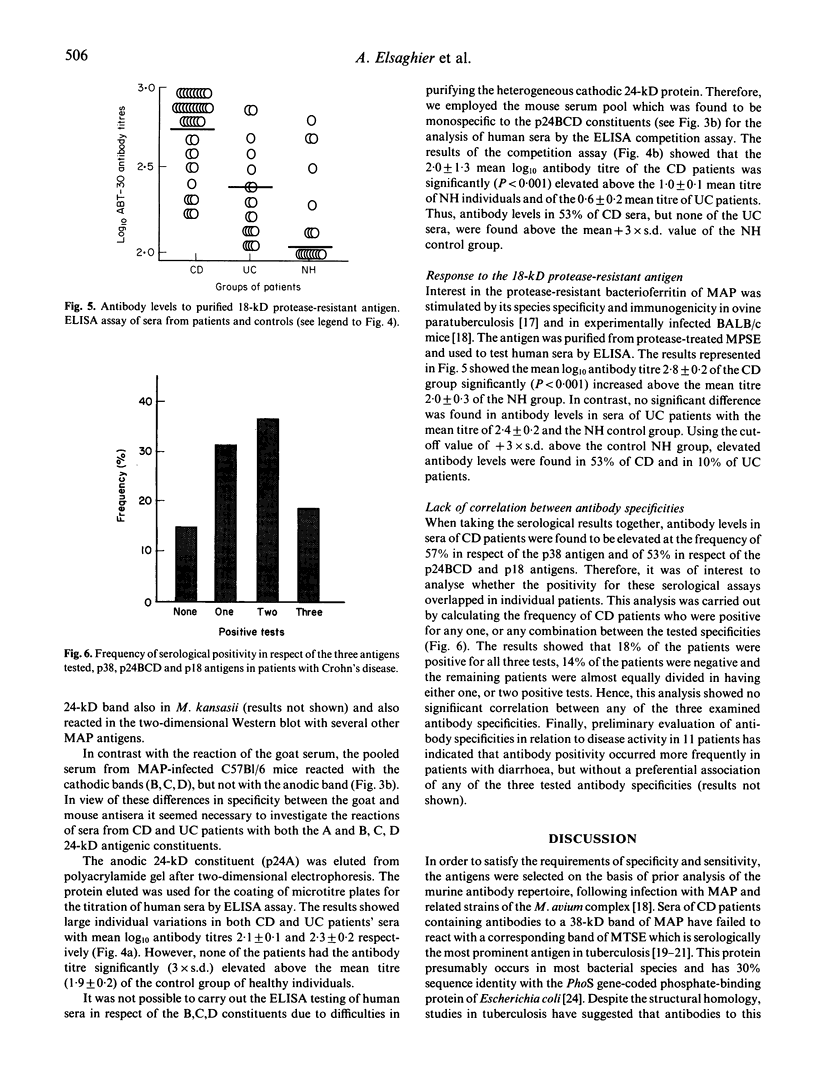
The possible role of infection with Mycobacterium paratuberculosis (MAP) for the etiopathogenesis of Crohn's disease (CD) has been a matter of long-term controversy. In addition to similarities with the pathology of ruminant paratuberculosis, DNA fingerprinting confirmed the organism isolated from gut tissue, but the specificity of the immune repertoire has not as yet been evaluated. We report here on a serological study of 29 patients with CD, 20 patients with ulcerative colitis and 18 healthy control subjects, using three antigens attributed with species-specificity and selective immunogenicity following MAP infection. Antibodies binding to the 38-kD band of MAP extract were demonstrable by the Western blot technique in 57% of CD patients. Antibody levels to the 24-kD (p24BCD) cathodic bands, determined by competition ELISA using a monospecific murine antiserum, and to the 18-kD protease-resistant purified bacterioferritin, detected by standard ELISA, were significantly elevated in 53% of CD patients. However, these three antibody specificities tested in individual CD patients did not show any correlation with each other. Thus, 18% of patients were positive for all three specificities, whilst 84% had antibodies to at least one of the specific antigens. Although the exact proportion of affected patients is yet to be defined, the serological results obtained support the view that MAP infection may play an etiological role in Crohn's disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Hansen E. B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989 Aug;57(8):2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G. H., Beck J. S., Schreuder G. M., D'Amaro J., de Vries R. R., Kardjito T., Ivanyi J. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J Infect Dis. 1989 Mar;159(3):549–555. doi: 10.1093/infdis/159.3.549. [DOI] [PubMed] [Google Scholar]
- Brooks B. W., Young N. M., Watson D. C., Robertson R. H., Sugden E. A., Nielsen K. H., Becker S. A. Mycobacterium paratuberculosis antigen D: characterization and evidence that it is a bacterioferritin. J Clin Microbiol. 1991 Aug;29(8):1652–1658. doi: 10.1128/jcm.29.8.1652-1658.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol Rev. 1989 Jan;2(1):90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S., Thayer W. R., Jr, Coutu J. A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984 Nov;20(5):966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. T., Høiby N., Jørgensen J. B., Bercovier H., Lambrecht R. S., Jørgensen E. Crossed immunoelectrophoretic analysis of Mycobacterium paratuberculosis. APMIS. 1991 Jan;99(1):83–92. [PubMed] [Google Scholar]
- Elsaghier A., Nolan A., Allen B., Ivanyi J. Distinctive western blot antibody patterns induced by infection of mice with individual strains of the Mycobacterium avium complex. Immunology. 1992 Jul;76(3):355–361. [PMC free article] [PubMed] [Google Scholar]
- Elsaghier A., Prantera C., Bothamley G., Wilkins E., Jindal S., Ivanyi J. Disease association of antibodies to human and mycobacterial hsp70 and hsp60 stress proteins. Clin Exp Immunol. 1992 Aug;89(2):305–309. doi: 10.1111/j.1365-2249.1992.tb06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K., Jackett P., Bothamley G. Immunological study of the defined constituents of mycobacteria. Springer Semin Immunopathol. 1988;10(4):279–300. doi: 10.1007/BF02053841. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. L. Johne's and Crohn's. Chronic inflammatory bowel diseases of infectious aetiology? Lancet. 1987 May 2;1(8540):1017–1019. doi: 10.1016/s0140-6736(87)92280-x. [DOI] [PubMed] [Google Scholar]
- Morris S. L., Rouse D. A., Hussong D., Chaparas S. D. Isolation and characterization of recombinant lambda gt11 bacteriophages expressing four different Mycobacterium intracellulare antigens. Infect Immun. 1990 Jan;58(1):17–20. doi: 10.1128/iai.58.1.17-20.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Prantera C., Bothamley G., Levenstein S., Mangiarotti R., Argentieri R. Crohn's disease and mycobacteria: two cases of Crohn's disease with high anti-mycobacterial antibody levels cured by dapsone therapy. Biomed Pharmacother. 1989;43(4):295–299. doi: 10.1016/0753-3322(89)90011-5. [DOI] [PubMed] [Google Scholar]
- Sanderson J. D., Moss M. T., Tizard M. L., Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut. 1992 Jul;33(7):890–896. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden E. A., Brooks B. W., Young N. M., Watson D. C., Nielsen K. H., Corner A. H., Turcotte C., Michaelides A., Stewart R. B. Chromatographic purification and characterization of antigens A and D from Mycobacterium paratuberculosis and their use in enzyme-linked immunosorbent assays for diagnosis of paratuberculosis in sheep. J Clin Microbiol. 1991 Aug;29(8):1659–1664. doi: 10.1128/jcm.29.8.1659-1664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Thorel M. F., Krichevsky M., Lévy-Frébault V. V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990 Jul;40(3):254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- Tsoulfa G., Rook G. A., Van-Embden J. D., Young D. B., Mehlert A., Isenberg D. A., Hay F. C., Lydyard P. M. Raised serum IgG and IgA antibodies to mycobacterial antigens in rheumatoid arthritis. Ann Rheum Dis. 1989 Feb;48(2):118–123. doi: 10.1136/ard.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins E. G., Ivanyi J. Potential value of serology for diagnosis of extrapulmonary tuberculosis. Lancet. 1990 Sep 15;336(8716):641–644. doi: 10.1016/0140-6736(90)92144-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Takahashi M., Fukasawa Y., Wada M., Abe C. Cloning and expression of the gene for the Avi-3 antigen of Mycobacterium avium and mapping of its epitopes. Infect Immun. 1992 Mar;60(3):1210–1216. doi: 10.1128/iai.60.3.1210-1216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]