Abstract
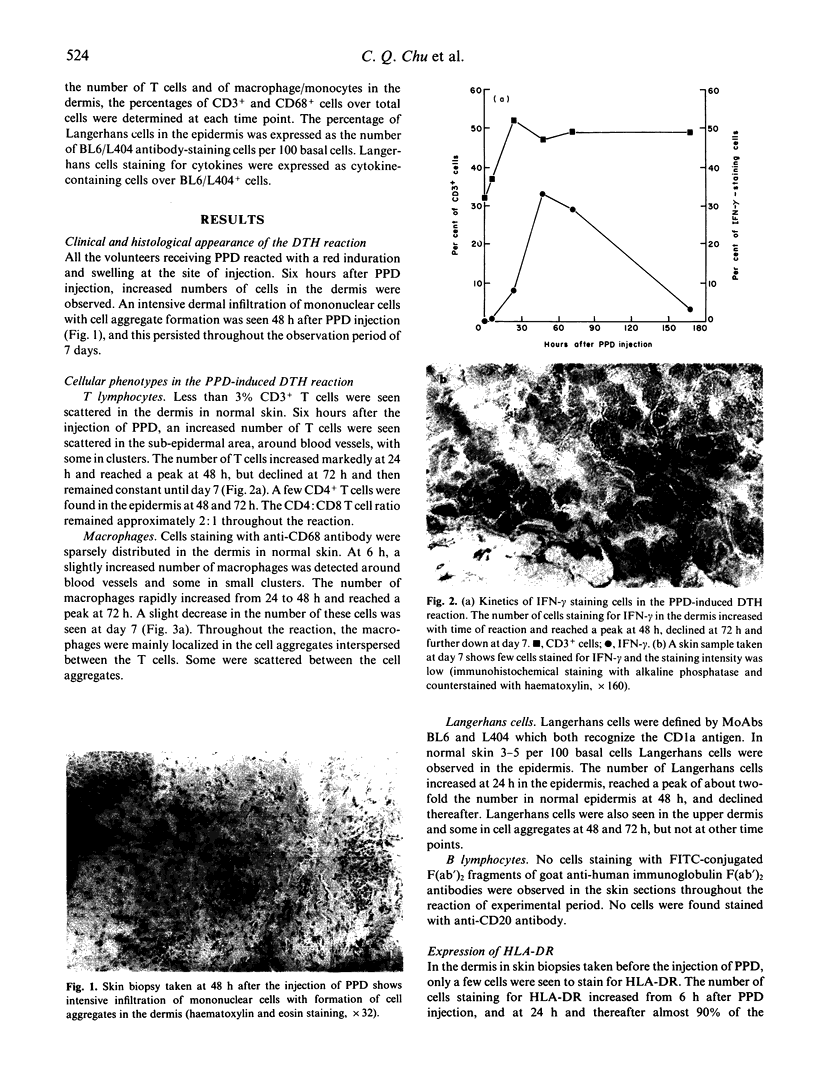
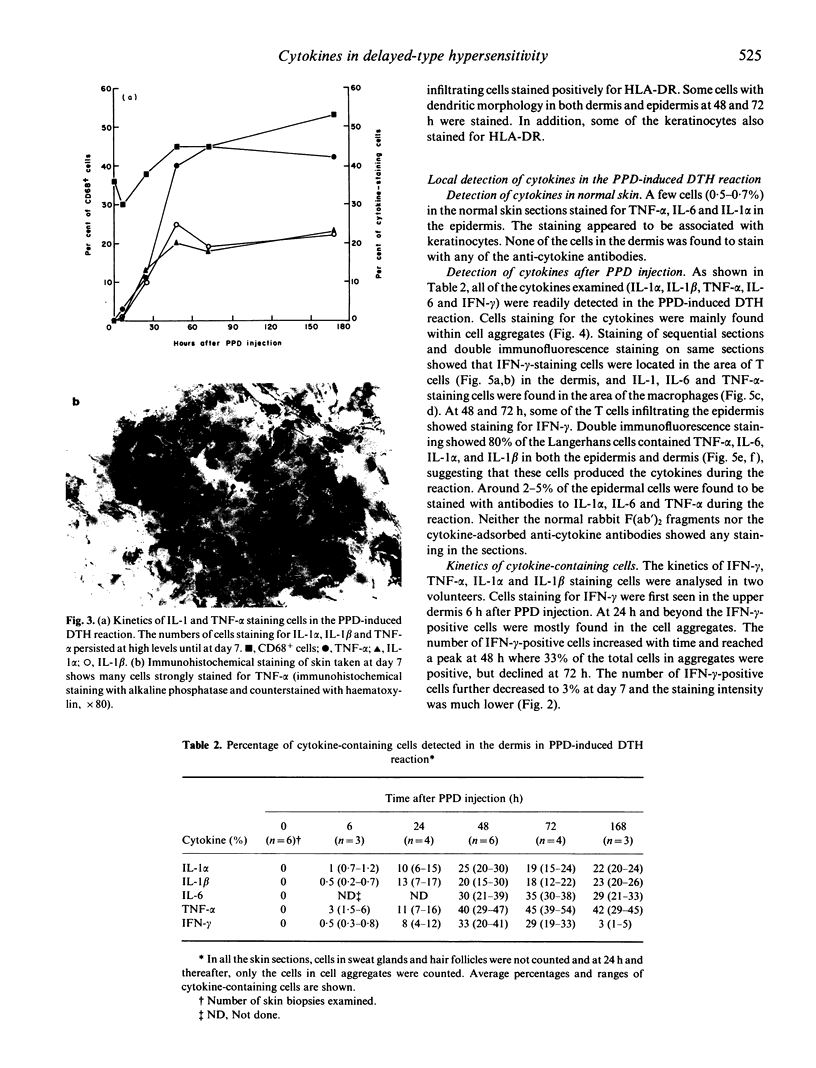
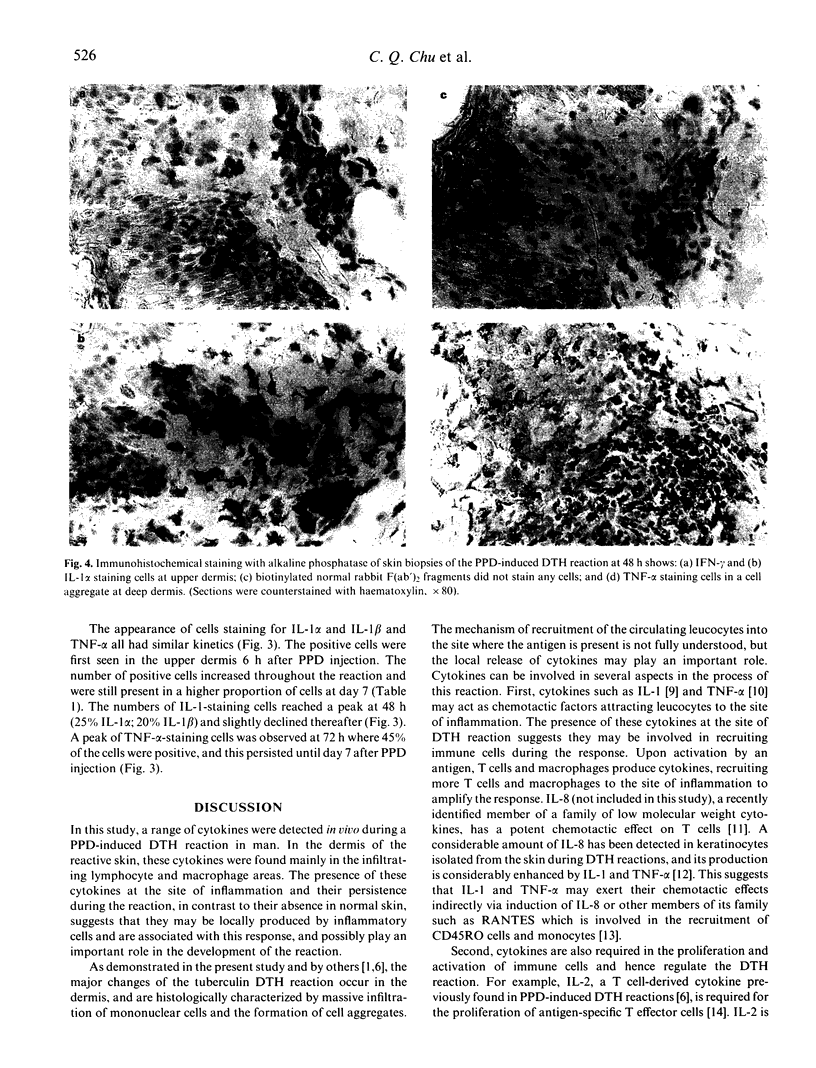
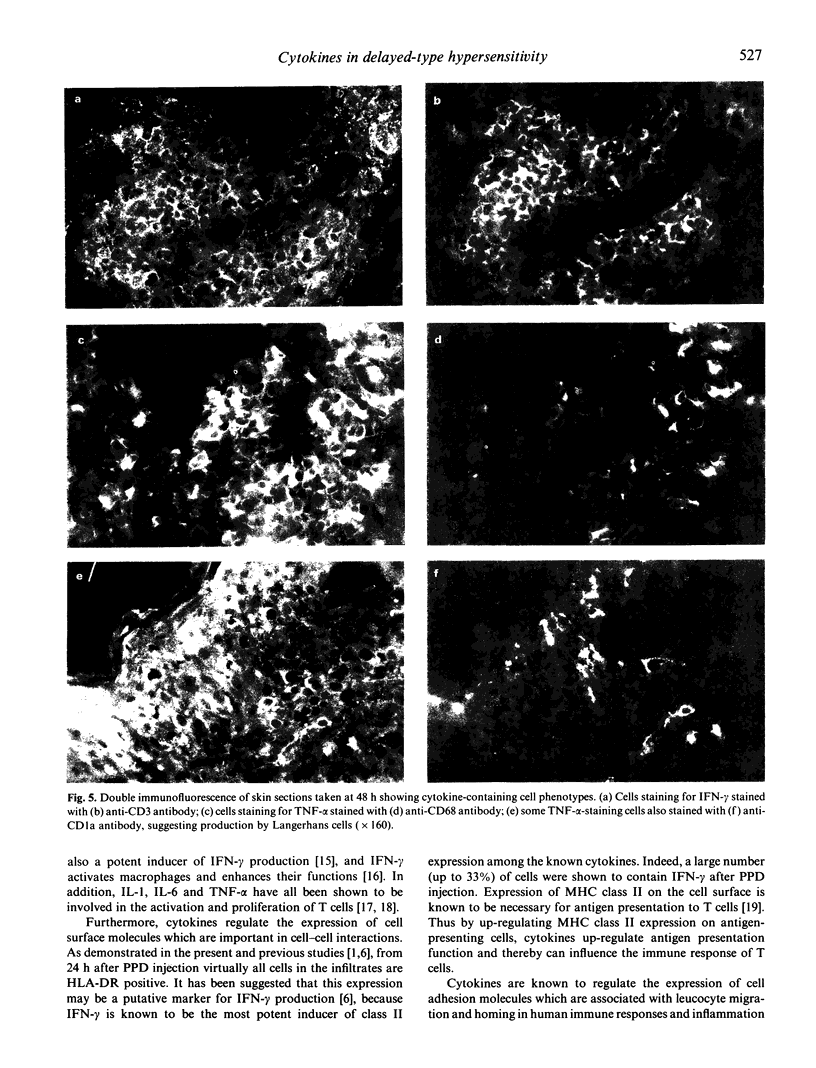
Cytokines are chiefly local mediators which play an important role in the regulation of the cell-cell interactions which may be involved in the development of the delayed-type hypersensitivity (DTH) reaction. Using immunohistochemical techniques, the presence of IL-1 alpha, IL-1 beta, IL-6, interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha) in the skin in tuberculin-purified protein derivative (PPD)-induced DTH reactions was investigated in six normal individuals. Cells staining for these cytokines were first observed 6 h after PPD challenge, and they were detected throughout the duration of the 7-day experiment. The number of cells staining for IFN-gamma reached a peak at 48 h, where 33% of the total aggregate cells were positive, but declined thereafter to 3% at day 7. On the other hand, the number of cells staining for TNF-alpha and IL-1 persisted at high levels throughout the observation period of 7 days (e.g. at 48 h and thereafter, about 40% cells positive for TNF-alpha and 20% for IL-1 alpha and IL-1 beta). Double immunofluorescence and staining on sequential sections showed that IFN-gamma-staining cells were CD3+ T cells; TNF-alpha, IL-1 and IL-6 staining cells were mainly of the CD68+ macrophages/monocytes and that 80% of the CD1a+ cells (Langerhans-like cells) in the dermis contained TNF-alpha and IL-1. The presence of these cytokines at the site of inflammation suggests that they may be locally produced by the inflammatory cells. Their persistence during the reaction suggests that they are intimately associated with this response, and are involved in the development of the reaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Allen M. H., MacDonald D. M. The effect of in vivo interferon-gamma on the distribution of LFA-1 and ICAM-1 in normal human skin. J Invest Dermatol. 1989 Oct;93(4):439–442. doi: 10.1111/1523-1747.ep12284016. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Field M., Chu C. Q., Feldmann M., Maini R. N. Cytokine expression in rheumatoid arthritis. Br J Rheumatol. 1991;30 (Suppl 1):76–80. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Demidem A., Taylor J. R., Grammer S. F., Streilein J. W. T-lymphocyte-activating properties of epidermal antigen-presenting cells from normal and psoriatic skin: evidence that psoriatic epidermal antigen-presenting cells resemble cultured normal Langerhans cells. J Invest Dermatol. 1991 Sep;97(3):454–460. doi: 10.1111/1523-1747.ep12481465. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Chu C., Feldmann M., Maini R. N. Interleukin-6 localisation in the synovial membrane in rheumatoid arthritis. Rheumatol Int. 1991;11(2):45–50. doi: 10.1007/BF00291144. [DOI] [PubMed] [Google Scholar]
- Fullmer M. A., Shen J. Y., Modlin R. L., Rea T. H. Immunohistological evidence of lymphokine production and lymphocyte activation antigens in tuberculin reactions. Clin Exp Immunol. 1987 Feb;67(2):383–390. [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. H., Ferguson J., Brown R. A., Kenicer K. J., Potts R. C., Coghill G., Swanson Beck J. Histometric study of the localisation of lymphocyte subsets and accessory cells in human Mantoux reactions. J Clin Pathol. 1984 Nov;37(11):1227–1234. doi: 10.1136/jcp.37.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf Y., Dinarello C. A., Yamin M., Sharon N., Milner Y., Gazit E. IL-1 production by T6 (CD1a) positive cord blood mononuclear cells (Langerhan's cell precursors?). Lymphokine Res. 1989 Winter;8(4):373–382. [PubMed] [Google Scholar]
- Hunninghake G. W., Glazier A. J., Monick M. M., Dinarello C. A. Interleukin-1 is a chemotactic factor for human T-lymphocytes. Am Rev Respir Dis. 1987 Jan;135(1):66–71. doi: 10.1164/arrd.1987.135.1.66. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., van der Meide P. The recruitment of lymphocytes into the skin by T cell lymphokines: the role of gamma-interferon. Clin Exp Immunol. 1988 Jul;73(1):70–75. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., vd Meide P. Lymphocyte recruitment in delayed-type hypersensitivity. The role of IFN-gamma. J Immunol. 1988 May 1;140(9):2989–2993. [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Konttinen Y. T., Bergroth V., Visa-Tolvanen K., Reitamo S., Förström L. Cellular infiltrate in situ and response kinetics of human intradermal and epicutaneous tuberculin reactions. Clin Immunol Immunopathol. 1983 Sep;28(3):441–449. doi: 10.1016/0090-1229(83)90111-3. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Morhenn V., Chiang Y. L., Shi T. Activated Langerhans cells release tumor necrosis factor. J Leukoc Biol. 1989 May;45(5):429–433. doi: 10.1002/jlb.45.5.429. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G. Leukocyte activating and chemotactic cytokines in cell-mediated immune reactions of the human skin. Acta Derm Venereol Suppl (Stockh) 1991;160:1–48. [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris P., Poston R. N., Thomas D. S., Thornhill M., Hawk J., Haskard D. O. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991 May;96(5):763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Seymour G. J., Duke O., Janossy G., Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982 Dec;74(2):358–369. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Sauder D. N., Dinarello C. A., Morhenn V. B. Langerhans cell production of interleukin-1. J Invest Dermatol. 1984 Jun;82(6):605–607. doi: 10.1111/1523-1747.ep12261439. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Scheynius A., Klareskog L., Forsum U. In situ identification of T lymphocyte subsets and HLA-DR expressing cells in the human skin tuberculin reaction. Clin Exp Immunol. 1982 Aug;49(2):325–330. [PMC free article] [PubMed] [Google Scholar]
- Shimada S., Katz S. I. The skin as an immunologic organ. Arch Pathol Lab Med. 1988 Mar;112(3):231–234. [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Zheng R. Q., Abney E. R., Chu C. Q., Field M., Maini R. N., Lamb J. R., Feldmann M. Detection of in vivo production of tumour necrosis factor-alpha by human thyroid epithelial cells. Immunology. 1992 Mar;75(3):456–462. [PMC free article] [PubMed] [Google Scholar]







