Abstract
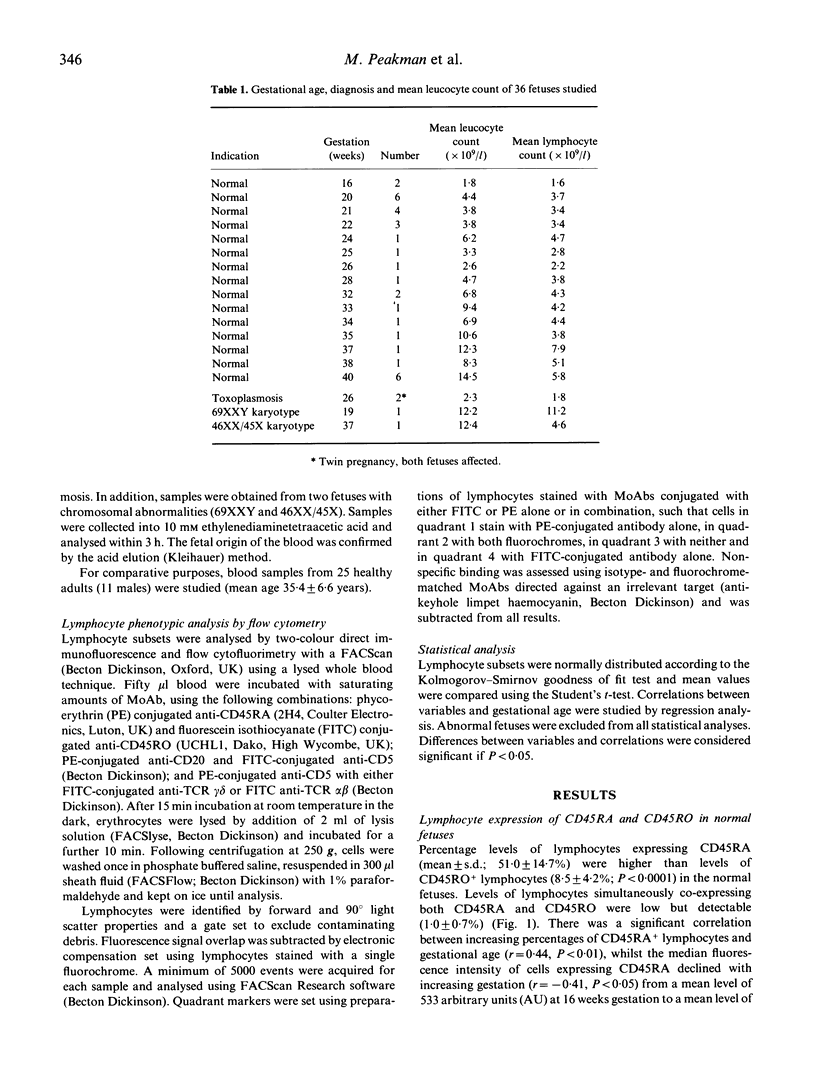
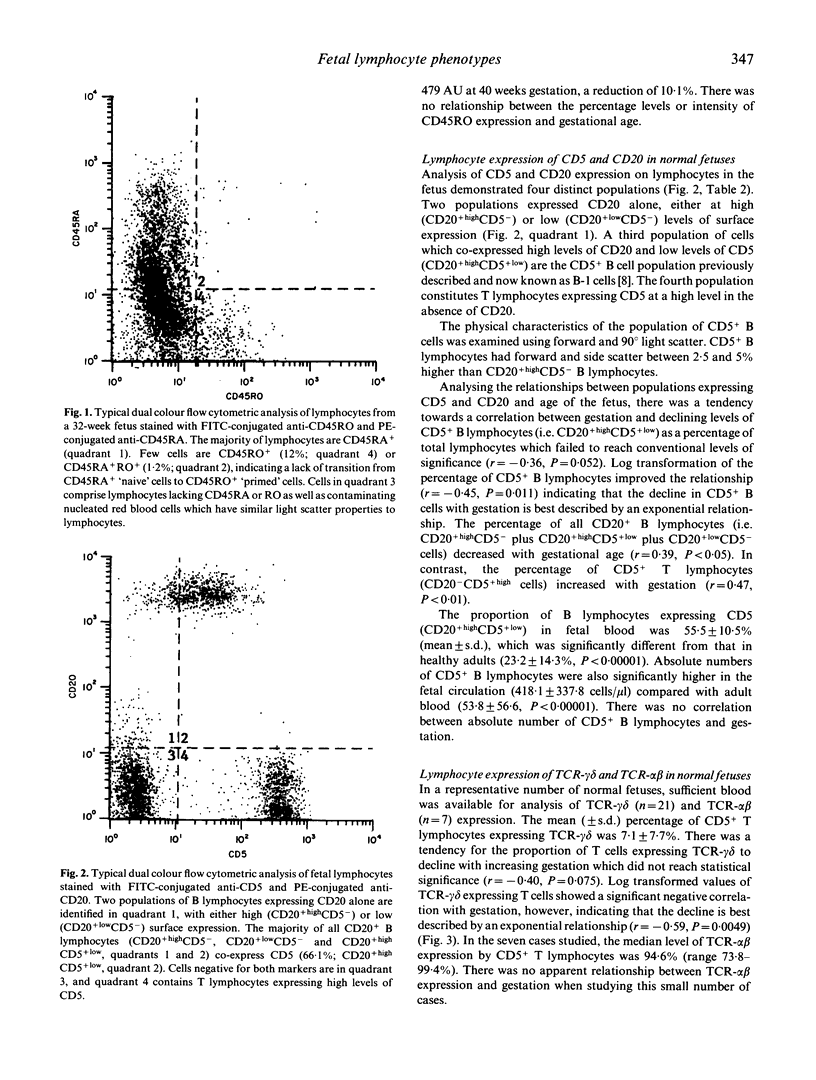
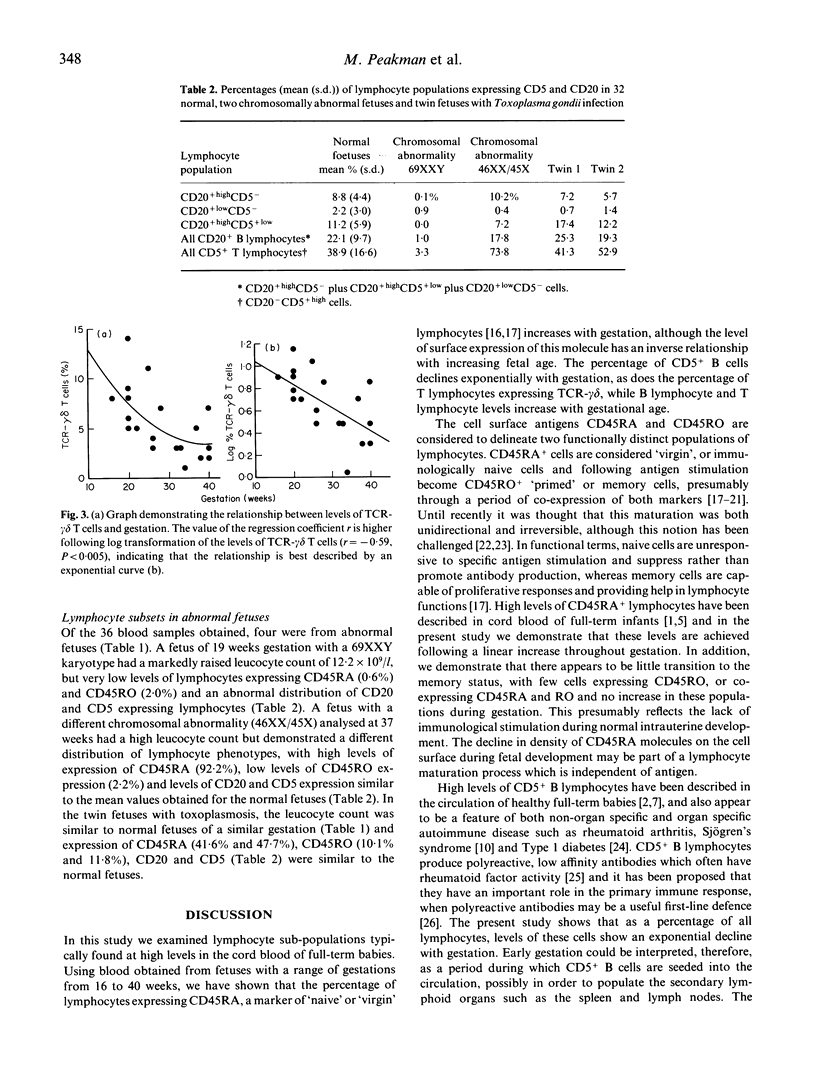
Using cord blood samples obtained from fetuses between 16 and 40 weeks gestation, we have used a lysed whole blood flow cytometric technique to study the natural history of lymphocyte phenotypes known to be highly represented in cord blood at birth. The majority (51.0 +/- 14.7%) of lymphocytes expressed CD45RA, a marker of 'virgin' cells and there was a correlation between increasing percentages of CD45RA+lymphocytes and gestational age (r = 0.44, P < 0.01). Few cells (8.5 +/- 4.2%) expressed the CD45RO marker of primed lymphocytes and very few (1.0 +/- 0.7%) co-expressed CD45RA and RO, indicating little traffic between the two maturation markers. The percentage of B lymphocytes co-expressing CD5 was high in the fetal circulation (55.5 +/- 10.5%) compared with healthy adults (23.2 +/- 14.3%; P < 0.00001) and the level of CD5+ B cells declined with gestational age in an exponential manner (r = -0.45, P < 0.05). Similarly, levels of T lymphocytes expressing the gamma delta T cell receptor (TCR) declined exponentially (r = -0.59, P < 0.005). These results demonstrate that lymphocytes remain almost entirely unprimed before birth. In addition, CD5+ B lymphocytes and TCR-gamma delta+ T lymphocytes decline exponentially towards birth, in a manner suggesting that they may be seeding peripheral sites such as the spleen, skin and mucosae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Antin J. H., Emerson S. G., Martin P., Gadol N., Ault K. A. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986 Jan;136(2):505–510. [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Bofill M., Janossy G., Janossa M., Burford G. D., Seymour G. J., Wernet P., Kelemen E. Human B cell development. II. Subpopulations in the human fetus. J Immunol. 1985 Mar;134(3):1531–1538. [PubMed] [Google Scholar]
- Brennan F., Plater-Zyberk C., Maini R. N., Feldmann M. Coordinate expansion of 'fetal type' lymphocytes (TCR gamma delta+T and CD5+B) in rheumatoid arthritis and primary Sjögren's syndrome. Clin Exp Immunol. 1989 Aug;77(2):175–178. [PMC free article] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989 Nov;10(11):364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Clement L. T., Vink P. E., Bradley G. E. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990 Jul 1;145(1):102–108. [PubMed] [Google Scholar]
- Clement L. T., Yamashita N., Martin A. M. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988 Sep 1;141(5):1464–1470. [PubMed] [Google Scholar]
- Davies N. P., Buggins A. G., Snijders R. J., Jenkins E., Layton D. M., Nicolaides K. H. Blood leucocyte count in the human fetus. Arch Dis Child. 1992 Apr;67(4 Spec No):399–403. doi: 10.1136/adc.67.4_spec_no.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele M., Foulon W., Renmans W., Segers E., Smet L., Jochmans K., Van Camp B. Hematologic values and lymphocyte subsets in fetal blood. Am J Clin Pathol. 1988 Jun;89(6):742–746. doi: 10.1093/ajcp/89.6.742. [DOI] [PubMed] [Google Scholar]
- Durandy A., Thuillier L., Forveille M., Fischer A. Phenotypic and functional characteristics of human newborns' B lymphocytes. J Immunol. 1990 Jan 1;144(1):60–65. [PubMed] [Google Scholar]
- Faustman D., Eisenbarth G., Daley J., Breitmeyer J. Abnormal T-lymphocyte subsets in type I diabetes. Diabetes. 1989 Nov;38(11):1462–1468. doi: 10.2337/diab.38.11.1462. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986 Oct;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986 Apr;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatani Y., Amino N., Kaneda T., Ichihara K., Tamaki H., Tachi J., Matsuzuka F., Fukata S., Kuma K., Miyai K. Marked increase of CD5 + B cells in hyperthyroid Graves' disease. Clin Exp Immunol. 1989 Nov;78(2):196–200. [PMC free article] [PubMed] [Google Scholar]
- Kantor A. A new nomenclature for B cells. Immunol Today. 1991 Nov;12(11):388–388. doi: 10.1016/0167-5699(91)90135-G. [DOI] [PubMed] [Google Scholar]
- Kipps T. J. The CD5 B cell. Adv Immunol. 1989;47:117–185. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- Kotylo P. K., Baenzinger J. C., Yoder M. C., Engle W. A., Bolinger C. D. Rapid analysis of lymphocyte subsets in cord blood. Am J Clin Pathol. 1990 Feb;93(2):263–266. doi: 10.1093/ajcp/93.2.263. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., LeCorre R., Mayo J., Bluestone J. A., Goodman T. Extrathymic selection of TCR gamma delta + T cells by class II major histocompatibility complex molecules. Cell. 1990 Oct 19;63(2):333–340. doi: 10.1016/0092-8674(90)90166-c. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Muñoz A., Gallart T., Viñas O., Gomis R. Increased CD5-positive B lymphocytes in type I diabetes. Clin Exp Immunol. 1991 Feb;83(2):304–308. doi: 10.1111/j.1365-2249.1991.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Burastero S. E., Notkins A. L., Casal P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J Immunol. 1988 Jun 15;140(12):4180–4186. [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Rainaut M., Pagniez M., Hercend T., Daffos F., Forestier F. Characterization of mononuclear cell subpopulations in normal fetal peripheral blood. Hum Immunol. 1987 Apr;18(4):331–337. doi: 10.1016/0198-8859(87)90079-6. [DOI] [PubMed] [Google Scholar]
- Richards S. J., Jones R. A., Roberts B. E., Patel D., Scott C. S. Relationships between 2H4 (CD45RA) and UCHL1 (CD45RO) expression by normal blood CD4+CD8-, CD4-CD8+, CD4-CD8dim+, CD3+CD4-CD8- and CD3-CD4-CD8- lymphocytes. Clin Exp Immunol. 1990 Jul;81(1):149–155. doi: 10.1111/j.1365-2249.1990.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein D. M., Yamada A., Schlossman S. F., Morimoto C. Cyclic regulation of CD45 isoform expression in a long term human CD4+CD45RA+ T cell line. J Immunol. 1991 Feb 15;146(4):1175–1183. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Serra H. M., Krowka J. F., Ledbetter J. A., Pilarski L. M. Loss of CD45R (Lp220) represents a post-thymic T cell differentiation event. J Immunol. 1988 Mar 1;140(5):1435–1441. [PubMed] [Google Scholar]


