Abstract
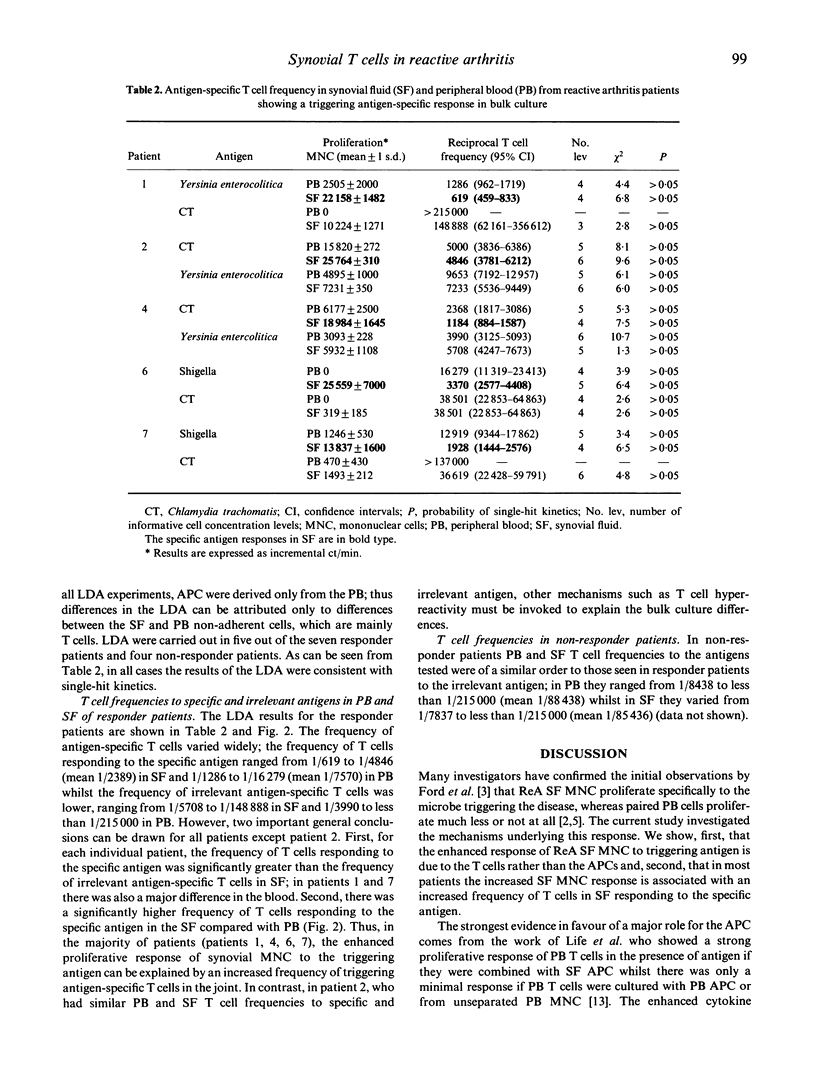
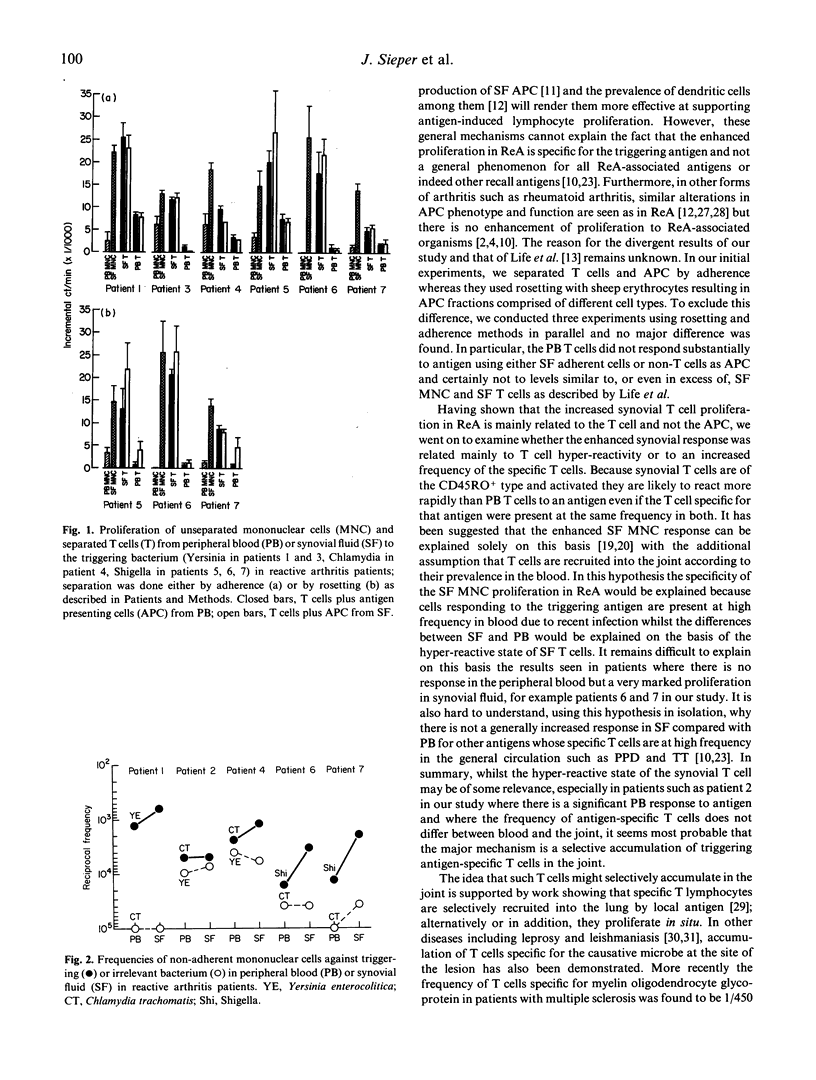
In reactive arthritis (ReA) there is specific proliferation of synovial fluid (SF) mononuclear cells (MNC) to the triggering bacterial antigen; comparatively little or no response is seen in peripheral blood (PB). To investigate the mechanism of this elevated local immune response, we examined patients with typical ReA who showed an enhanced antigen-specific synovial immune response in bulk culture. Using separated fractions of T cells and antigen-presenting cells (APC) from PB and SF we showed that the synovial T cells rather than SF APC are responsible for the specific proliferation. By limiting dilution analysis, the frequency of T cells responding to the specific antigen was found to be significantly increased compared with the frequency of irrelevant antigen-specific T cells. Furthermore, the frequency of T cells responding to the specific antigen was higher in SF (between 1/619 and 1/4846, mean 1/2389) than in PB (between 1/1286 and 1/16,279, mean 1/7350). We conclude that the specific synovial cellular immune response in ReA is mainly due to an expansion of antigen-specific T cells within the joint. However, the non-specific hyper-reactivity of SF T cells and differences between SF and PB APC may make a more minor contribution.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. Immunological memory in T cells. Curr Opin Immunol. 1991 Jun;3(3):355–360. doi: 10.1016/0952-7915(91)90038-3. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Sarov I., Rumpianesi F., Donati M., Melega C., Varotti C., La Placa M. Serum specific IgA antibody to Chlamydia trachomatis in patients with chlamydial infections detected by ELISA and an immunofluorescence test. J Clin Pathol. 1984 Jun;37(6):686–691. doi: 10.1136/jcp.37.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. I. A cytofluorographic study of monocyte differentiation antigens and class II antigens and their regulation by gamma-interferon. Arthritis Rheum. 1987 Aug;30(8):857–863. doi: 10.1002/art.1780300803. [DOI] [PubMed] [Google Scholar]
- Ford D. K., da Roza D. M., Shah P., Wenman W. M. Cell-mediated immune responses of synovial mononuclear cells in Reiter's syndrome against ureaplasmal and chlamydial antigens. J Rheumatol. 1980 Sep-Oct;7(5):751–755. [PubMed] [Google Scholar]
- Ford D. K., da Roza D., Schulzer M. Lymphocytes from the site of disease but not blood lymphocytes indicate the cause of arthritis. Ann Rheum Dis. 1985 Oct;44(10):701–710. doi: 10.1136/ard.44.10.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Tsuyuguchi I. Frequency of tuberculin-reactive T-lymphocytes in pleural fluid and blood from patients with tuberculous pleurisy. Chest. 1986 Apr;89(4):530–532. doi: 10.1378/chest.89.4.530. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Granfors K., Merilahti-Palo R., Bailey L., Consalvey S., Toivanen A., Bacon P. A. Synovial T lymphocyte recognition of organisms that trigger reactive arthritis. Clin Exp Immunol. 1989 Jun;76(3):348–353. [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., Lindberg A. A., Mäki-Ikola O., von Essen R., Lahesmaa-Rantala R., Isomäki H., Saario R., Arnold W. J., Toivanen A. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990 Mar 24;335(8691):685–688. doi: 10.1016/0140-6736(90)90804-e. [DOI] [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., von Essen R., Lahesmaa-Rantala R., Isomäki O., Pekkola-Heino K., Merilahti-Palo R., Saario R., Isomäki H., Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989 Jan 26;320(4):216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- Hammer M., Zeidler H., Klimsa S., Heesemann J. Yersinia enterocolitica in the synovial membrane of patients with Yersinia-induced arthritis. Arthritis Rheum. 1990 Dec;33(12):1795–1800. doi: 10.1002/art.1780331206. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Eggers C., Schröder J. Serological diagnosis of yersiniosis by immunoblot technique using virulence-associated antigen of enteropathogenic Yersiniae. Contrib Microbiol Immunol. 1987;9:285–289. [PubMed] [Google Scholar]
- Keat A. C., Knight S. C. Do synovial fluid cells indicate the cause of reactive arthritis? J Rheumatol. 1990 Oct;17(10):1257–1259. [PubMed] [Google Scholar]
- Keat A. Reiter's syndrome and reactive arthritis in perspective. N Engl J Med. 1983 Dec 29;309(26):1606–1615. doi: 10.1056/NEJM198312293092604. [DOI] [PubMed] [Google Scholar]
- Keat A., Thomas B., Dixey J., Osborn M., Sonnex C., Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987 Jan 10;1(8524):72–74. doi: 10.1016/s0140-6736(87)91910-6. [DOI] [PubMed] [Google Scholar]
- Kingsley G. H., Pitzalis C., Panayi G. S. Abnormal lymphocyte reactivity to self-major histocompatibility antigens in rheumatoid arthritis. J Rheumatol. 1987 Aug;14(4):667–673. [PubMed] [Google Scholar]
- Kingsley G., Pitzalis C., Kyriazis N., Panayi G. S. Abnormal helper-inducer/suppressor-inducer T-cell subset distribution and T-cell activation status are common to all types of chronic synovitis. Scand J Immunol. 1988 Aug;28(2):225–232. doi: 10.1111/j.1365-3083.1988.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Life P. F., Viner N. J., Bacon P. A., Gaston J. S. Synovial fluid antigen-presenting cells unmask peripheral blood T cell responses to bacterial antigens in inflammatory arthritis. Clin Exp Immunol. 1990 Feb;79(2):189–194. doi: 10.1111/j.1365-2249.1990.tb05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightstone E., Marvel J., Mitchison A. Memory in helper T cells of minor histocompatibility antigens, revealed in vivo by alloimmunizations in combination with Thy-1 antigen. Eur J Immunol. 1992 Jan;22(1):115–122. doi: 10.1002/eji.1830220118. [DOI] [PubMed] [Google Scholar]
- Lipscomb M. F., Lyons C. R., O'Hara R. M., Jr, Stein-Streilein J. The antigen-induced selective recruitment of specific T lymphocytes to the lung. J Immunol. 1982 Jan;128(1):111–115. [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. Specialization, tolerance, memory, competition, latency, and strife among T cells. Annu Rev Immunol. 1992;10:1–12. doi: 10.1146/annurev.iy.10.040192.000245. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Melancon-Kaplan J., Young S. M., Pirmez C., Kino H., Convit J., Rea T. H., Bloom B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A., Schlesier M., Schneider H., Vogt A., Peter H. H. Frequencies of Borrelia burgdorferi-reactive T lymphocytes in Lyme arthritis. Rheumatol Int. 1989;9(3-5):237–241. doi: 10.1007/BF00271888. [DOI] [PubMed] [Google Scholar]
- Nordström D., Konttinen Y. T., Bergroth V., Leirisalo-Repo M. Synovial fluid cells in Reiter's syndrome. Ann Rheum Dis. 1985 Dec;44(12):852–856. doi: 10.1136/ard.44.12.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley M. G., Kingsley G., Pitzalis C., Panayi G. S. Monocyte activation in rheumatoid arthritis: evidence for in situ activation and differentiation in joints. Br J Rheumatol. 1990 Apr;29(2):84–88. doi: 10.1093/rheumatology/29.2.84. [DOI] [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Schönberg A., Camey C., Kahl O., Wilske B., Preac-Mursic V., Hovind-Hougen K. First isolation of Borrelia burgdorferi, the agent of Lyme borreliosis, from Ixodes ricinus (Acari: Ixodidae) in Berlin (West). Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jun;268(4):487–494. doi: 10.1016/s0176-6724(88)80128-7. [DOI] [PubMed] [Google Scholar]
- Sieper J., Braun J., Brandt J., Miksits K., Heesemann J., Laitko S., Sörensen H., Distler A., Kingsley G. Pathogenetic role of Chlamydia, Yersinia and Borrelia in undifferentiated oligoarthritis. J Rheumatol. 1992 Aug;19(8):1236–1242. [PubMed] [Google Scholar]
- Sieper J., Kingsley G., Palacios-Boix A., Pitzalis C., Treharne J., Hughes R., Keat A., Panayi G. S. Synovial T lymphocyte-specific immune response to Chlamydia trachomatis in Reiter's disease. Arthritis Rheum. 1991 May;34(5):588–598. doi: 10.1002/art.1780340511. [DOI] [PubMed] [Google Scholar]
- Stagg A. J., Harding B., Hughes R. A., Keat A., Knight S. C. The distribution and functional properties of dendritic cells in patients with seronegative arthritis. Clin Exp Immunol. 1991 Apr;84(1):66–71. doi: 10.1111/j.1365-2249.1991.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Link H., Olsson T., Xiao B. G., Andersson G., Ekre H. P., Linington C., Diener P. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J Immunol. 1991 Mar 1;146(5):1490–1495. [PubMed] [Google Scholar]
- Ziff M. Role of the endothelium in chronic inflammatory synovitis. Arthritis Rheum. 1991 Nov;34(11):1345–1352. doi: 10.1002/art.1780341102. [DOI] [PubMed] [Google Scholar]


