Abstract
A phenotypic analysis of bronchoalveolar lavage fluid (BALF) and peripheral blood (PB) cells in maedi visna virus (MVV)-infected sheep has been performed. The differential cell count in BALF from MVV-infected animals was characterized by a significant increase (P < 0.05) in lymphocytes and neutrophils. Lymphocyte phenotyping in BALF from MVV-infected sheep showed a significant decrease (P < 0.05) of CD4+ cells, a significant increase (P < 0.05) of CD8+ cells and a significant inversion (P < 0.001) of the CD4+/CD8+ ratio. CD5+ lymphocytes were also significantly decreased (P < 0.05). Gamma delta T cells and B cells did not differ significantly when compared with the controls. No correlation was observed between BALF and PB lymphocyte phenotypes. BALF macrophages from MVV-infected animals showed increased MHC class II expression and BALF lymphocytes from the same animals demonstrated up-regulation of LFA-1 and LFA-3 expression. These findings and their relationship with lentiviral pathogenesis are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beya M. F., Miyasaka M., Dudler L., Ezaki T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1986 Jan;57(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- Cordier G., Cozon G., Greenland T., Rocher F., Guiguen F., Guerret S., Brune J., Mornex J. F. In vivo activation of alveolar macrophages in ovine lentivirus infection. Clin Immunol Immunopathol. 1990 Jun;55(3):355–367. doi: 10.1016/0090-1229(90)90124-9. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Schmerr M. J., Brogden K. A. Ovine progressive pneumonia (maedi-visna) in sheep. Vet Microbiol. 1988 Jul;17(3):237–250. doi: 10.1016/0378-1135(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Wood R. L., Brogden K. A. Arthritis associated with ovine progressive pneumonia. Am J Vet Res. 1985 Jan;46(1):65–68. [PubMed] [Google Scholar]
- Dalziel R. G., Hopkins J., Watt N. J., Dutia B. M., Clarke H. A., McConnell I. Identification of a putative cellular receptor for the lentivirus visna virus. J Gen Virol. 1991 Aug;72(Pt 8):1905–1911. doi: 10.1099/0022-1317-72-8-1905. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Macher A. M., Longo D. L., Lane H. C., Rook A. H., Masur H., Gelmann E. P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984 Jan;100(1):92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- Georgsson G., Pálsson P. A. The histopathology of maedi a slow, viral pneumonia of sheep. Vet Pathol. 1971;8(1):63–80. doi: 10.1177/030098587100800108. [DOI] [PubMed] [Google Scholar]
- Gorrell M. D., Brandon M. R., Sheffer D., Adams R. J., Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992 May;66(5):2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkiss G. D., Watt N. J., King T. J., Williams J., Hopkins J. Retroviral arthritis: phenotypic analysis of cells in the synovial fluid of sheep with inflammatory synovitis associated with visna virus infection. Clin Immunol Immunopathol. 1991 Jul;60(1):106–117. doi: 10.1016/0090-1229(91)90116-r. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Mackay C. R. Prominence of gamma delta T cells in the ruminant immune system. Immunol Today. 1991 Jan;12(1):30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- Hopkins J., Dutia B. M. Monoclonal antibodies to the sheep analogues of human CD45 (leucocyte common antigen), MHC class I and CD5. Differential expression after lymphocyte activation in vivo. Vet Immunol Immunopathol. 1990 Apr;24(4):331–346. doi: 10.1016/0165-2427(90)90004-c. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Hünig T., Mitnacht R., Tiefenthaler G., Köhler C., Miyasaka M. T11TS, the cell surface molecule binding to the "erythrocyte receptor" of T lymphocytes: cellular distribution, purification to homogeneity and biochemical properties. Eur J Immunol. 1986 Dec;16(12):1615–1621. doi: 10.1002/eji.1830161223. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Zink C., Narayan O. Pathogenesis of ovine lentivirus-induced arthritis: phenotypic evaluation of T lymphocytes in synovial fluid, synovium, and peripheral circulation. Clin Immunol Immunopathol. 1989 Aug;52(2):323–330. doi: 10.1016/0090-1229(89)90183-9. [DOI] [PubMed] [Google Scholar]
- Kennedy P. G., Narayan O., Ghotbi Z., Hopkins J., Gendelman H. E., Clements J. E. Persistent expression of Ia antigen and viral genome in visna-maedi virus-induced inflammatory cells. Possible role of lentivirus-induced interferon. J Exp Med. 1985 Dec 1;162(6):1970–1982. doi: 10.1084/jem.162.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Butera S. T., Callahan G. N., DeMartini J. C. Spontaneous interferon production by pulmonary leukocytes is associated with lentivirus-induced lymphoid interstitial pneumonia. J Immunol. 1988 Feb 1;140(3):779–785. [PubMed] [Google Scholar]
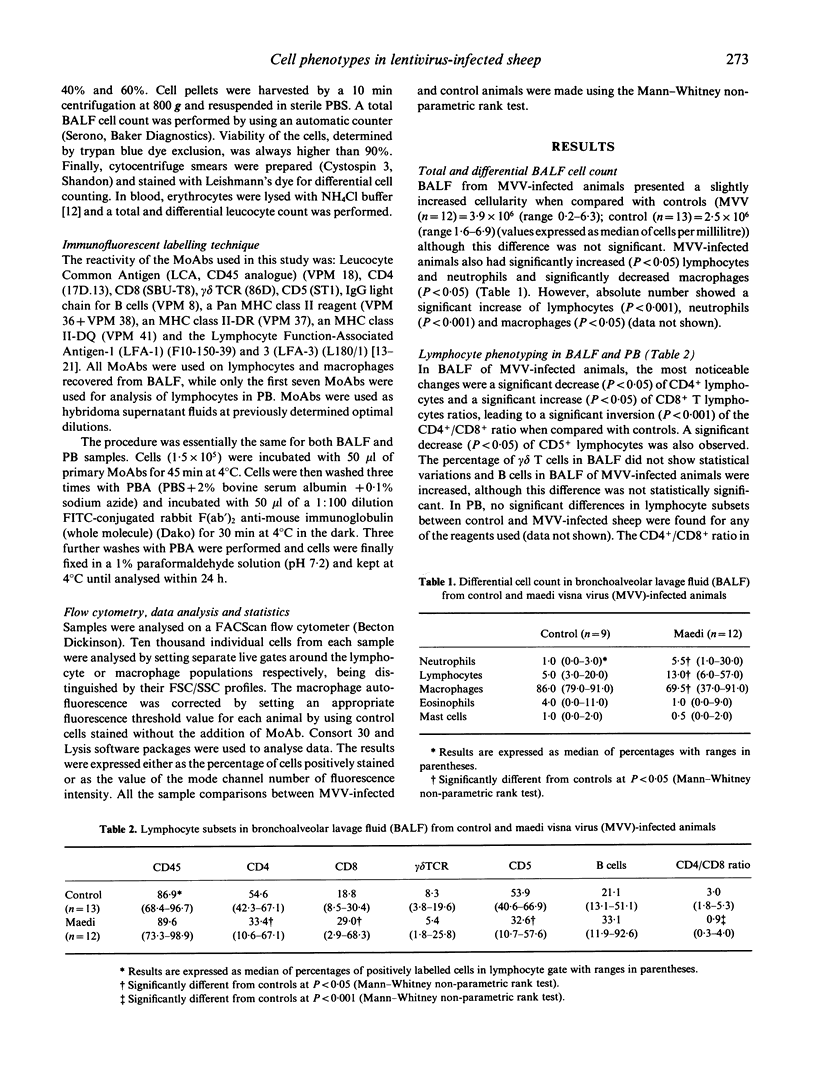
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Hein W. R., Brown M. H., Matzinger P. Unusual expression of CD2 in sheep: implications for T cell interactions. Eur J Immunol. 1988 Nov;18(11):1681–1688. doi: 10.1002/eji.1830181105. [DOI] [PubMed] [Google Scholar]
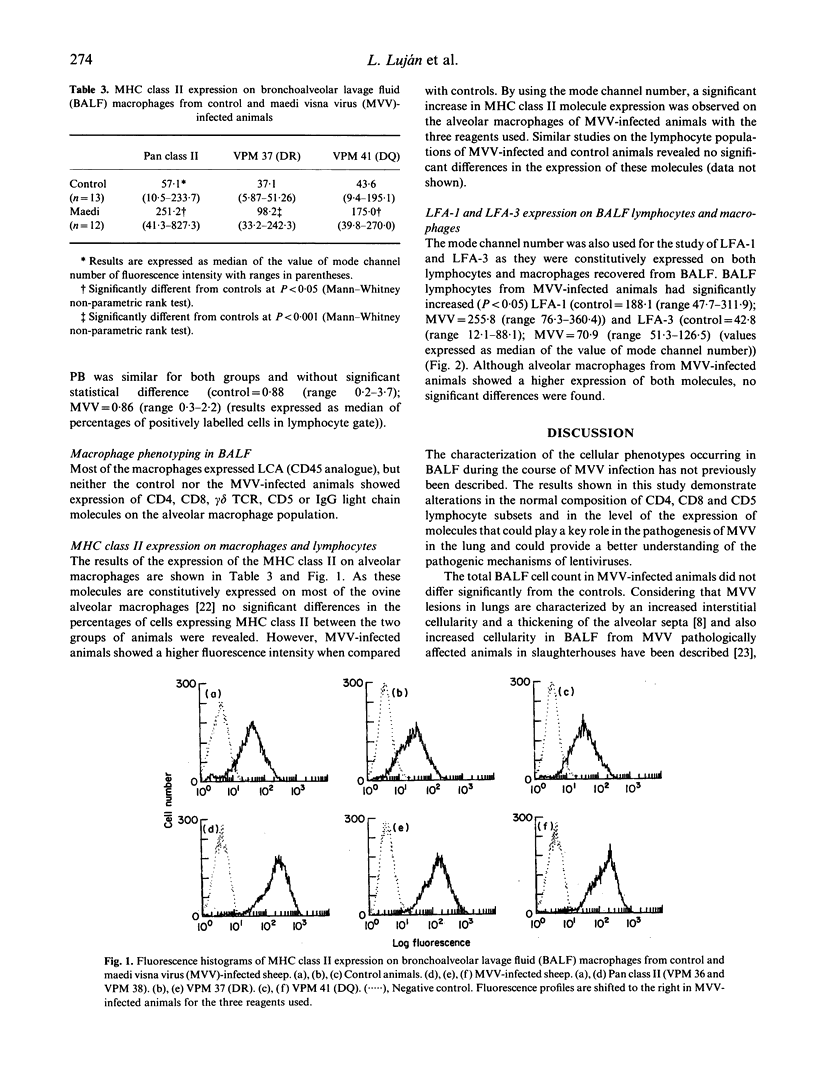
- Mackay C. R., Marston W. L., Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990 Mar 1;171(3):801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Williams D. E., Dines D. E., Sanderson D. R. Interstitial lung disease. Assessment by bronchoalveolar lavage. Mayo Clin Proc. 1983 Nov;58(11):751–757. [PubMed] [Google Scholar]
- Narayan O., Clements J. E. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989 Jul;70(Pt 7):1617–1639. doi: 10.1099/0022-1317-70-7-1617. [DOI] [PubMed] [Google Scholar]
- Nash A. D., Barcham G. J., Andrews A. E., Brandon M. R. Characterisation of ovine alveolar macrophages: regulation of surface antigen expression and cytokine production. Vet Immunol Immunopathol. 1992 Feb 15;31(1-2):77–94. doi: 10.1016/0165-2427(92)90088-8. [DOI] [PubMed] [Google Scholar]
- Pardi R., Inverardi L., Bender J. R. Regulatory mechanisms in leukocyte adhesion: flexible receptors for sophisticated travelers. Immunol Today. 1992 Jun;13(6):224–230. doi: 10.1016/0167-5699(92)90159-5. [DOI] [PubMed] [Google Scholar]
- Peluso R., Haase A., Stowring L., Edwards M., Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985 Nov;147(1):231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P. A. Visna of sheep; a slow, demyelinating infection. Br J Exp Pathol. 1958 Oct;39(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- Scott G. B., Buck B. E., Leterman J. G., Bloom F. L., Parks W. P. Acquired immunodeficiency syndrome in infants. N Engl J Med. 1984 Jan 12;310(2):76–81. doi: 10.1056/NEJM198401123100202. [DOI] [PubMed] [Google Scholar]
- Venet A., Clavel F., Israël-Biet D., Rouzioux C., Dennewald G., Stern M., Vittecoq D., Régnier B., Cayrol E., Chrétien J. Lung in acquired immune deficiency syndrome: infectious and immunological status assessed by bronchoalveolar lavage. Bull Eur Physiopathol Respir. 1985 Nov-Dec;21(6):535–543. [PubMed] [Google Scholar]
- Watt N. J., King T. J., Collie D., McIntyre N., Sargan D., McConnell I. Clinicopathological investigation of primary, uncomplicated maedi-visna virus infection. Vet Rec. 1992 Nov 14;131(20):455–461. doi: 10.1136/vr.131.20.455. [DOI] [PubMed] [Google Scholar]
- Westermann J., Pabst R. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today. 1990 Nov;11(11):406–410. doi: 10.1016/0167-5699(90)90160-b. [DOI] [PubMed] [Google Scholar]
- Winward L. D., Leendertsen L., Shen D. T. Microimmunodiffusion test for diagnosis of ovine progressive pneumonia. Am J Vet Res. 1979 Apr;40(4):564–566. [PubMed] [Google Scholar]
- van der Molen E. J., Vecht U., Houwers D. J. A chronic indurative mastitis in sheep, associated with maedi/visna virus infection. Vet Q. 1985 Apr;7(2):112–119. doi: 10.1080/01652176.1985.9693966. [DOI] [PubMed] [Google Scholar]


