Abstract
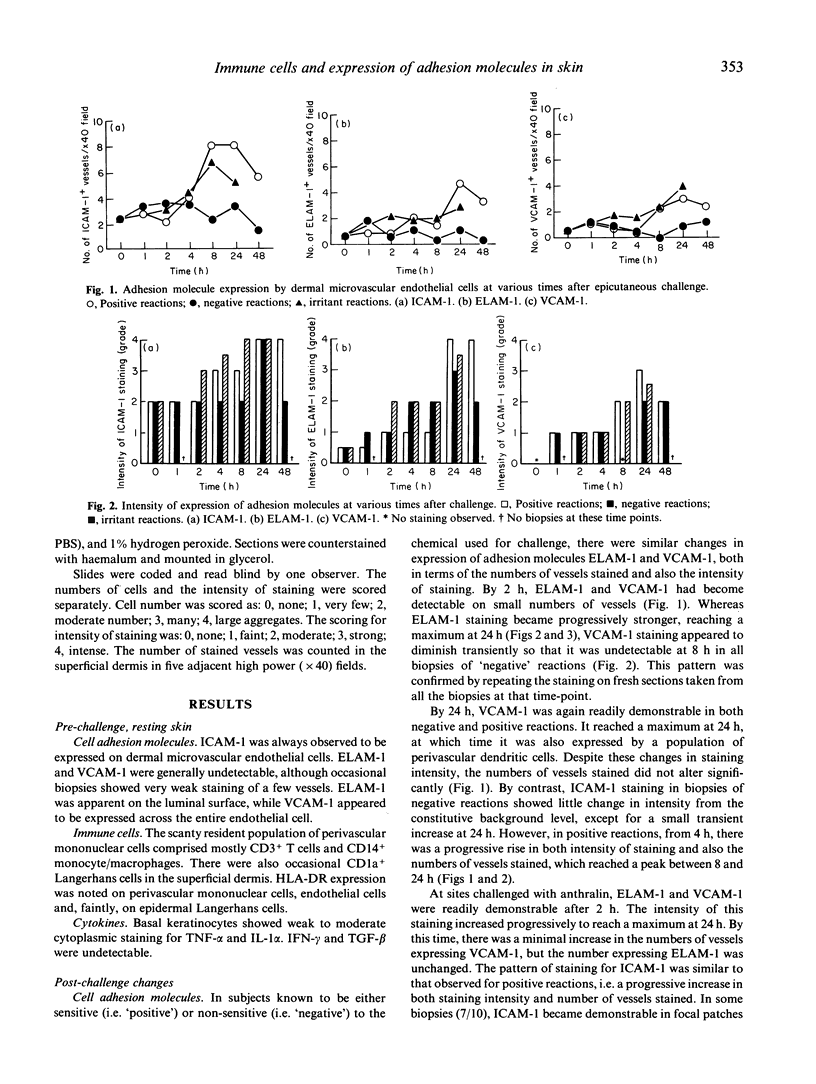
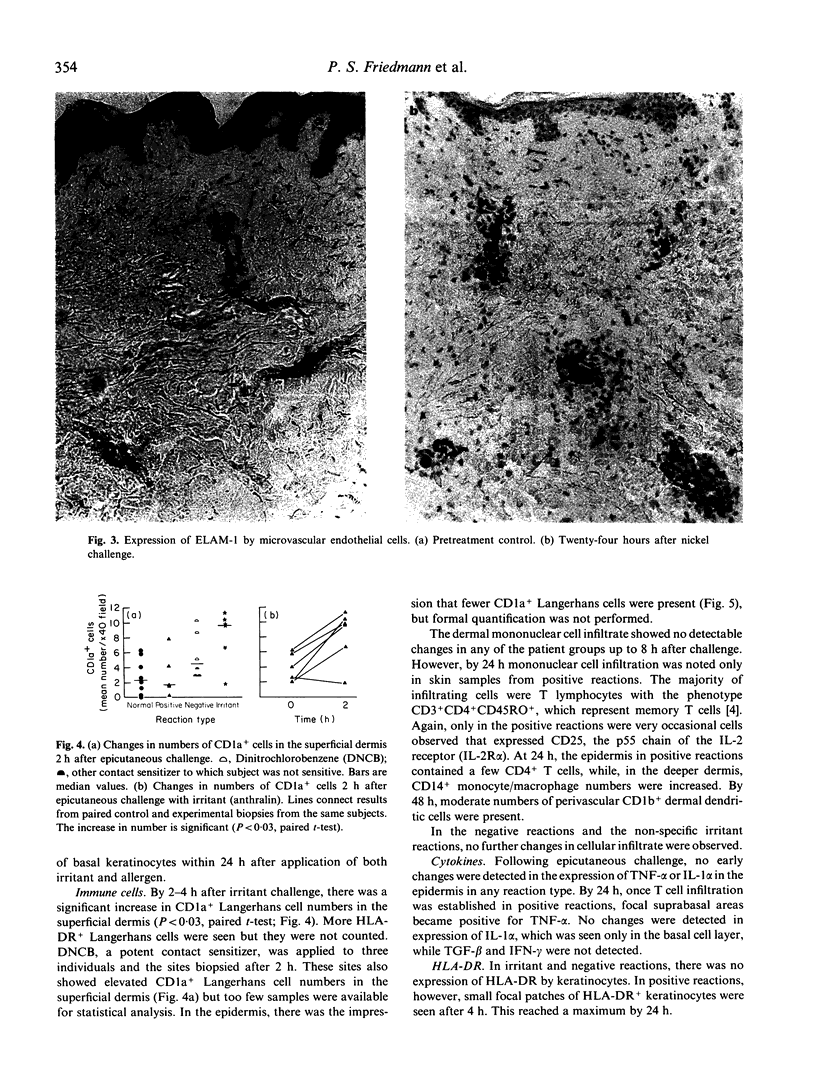
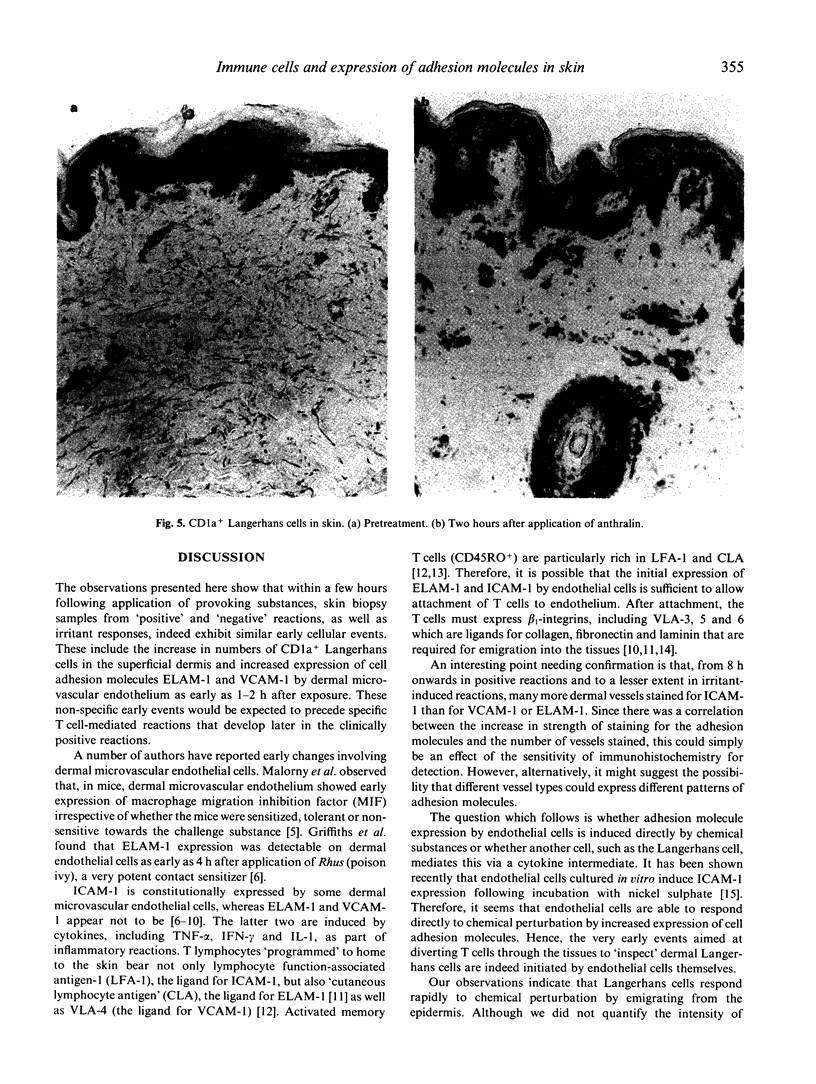
As part of the defence function of skin it seems probable that mechanisms exist for the rapid recruitment of immune surveillance to 'inspect' any foreign substance that penetrates the skin. In the present study, evidence of such mechanisms was sought by following the time course of early changes in distribution of immune cells, expression of cell adhesion molecules and cytokines after epicutaneous challenge with provoking chemicals to which subjects were known to be either specifically 'sensitive' or 'non-sensitive'; anthralin, an irritant chemical, was used as control. Fifty-seven individuals were studied and there were at least five biopsy samples at each time point. Regardless of whether individuals were sensitive or not, or of the type of chemical, dermal microvascular endothelial cells showed increased expression of the adhesion molecules ELAM-1 and VCAM-1 within 2 h, and ICAM-1 within 8 h. The intensity of immunohistochemical staining increased progressively up to 24 h. More vessels stained for ICAM-1 than for VCAM-1 or ELAM-1, implying that not every vessel expressed all three cell adhesion molecules. Another early change, observed 2 h after irritant challenge, was a significant increase in numbers of CD1a+ dendritic cells in the superficial dermis from a median of 3/high power field (hpf) to 9.5/hpf (P < 0.03). This was not observed with 'weak' provoking substances, such as nickel, but did occur with the potent provoking agent dinitrochlorobenzene (DNCB). Thus, as little as 2 h after contact with provoking chemicals, the skin activates cellular mechanisms to increase T cell infiltration for the presumed purpose of immune surveillance. These mechanisms are not dependent upon specific immune sensitivity and reflect a capacity of skin cells to respond to chemical provocation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba S., Katz S. I. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990 Nov 1;145(9):2791–2796. [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Becker D., Neiss U., Neis S., Reske K., Knop J. Contact allergens modulate the expression of MHC class II molecules on murine epidermal Langerhans cells by endocytotic mechanisms. J Invest Dermatol. 1992 May;98(5):700–705. doi: 10.1111/1523-1747.ep12499912. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Friedmann P. S., Moss C., Shuster S., Simpson J. M. Quantitative relationships between sensitizing dose of DNCB and reactivity in normal subjects. Clin Exp Immunol. 1983 Sep;53(3):709–715. [PMC free article] [PubMed] [Google Scholar]
- Griffiths C. E., Barker J. N., Kunkel S., Nickoloff B. J. Modulation of leucocyte adhesion molecules, a T-cell chemotaxin (IL-8) and a regulatory cytokine (TNF-alpha) in allergic contact dermatitis (rhus dermatitis). Br J Dermatol. 1991 Jun;124(6):519–526. doi: 10.1111/j.1365-2133.1991.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Malorny U., Knop J., Burmeister G., Sorg C. Immunohistochemical demonstration of migration inhibitory factor (MIF) in experimental allergic contact dermatitis. Clin Exp Immunol. 1988 Jan;71(1):164–170. [PMC free article] [PubMed] [Google Scholar]
- Misch K., Davies M., Greaves M., Coutts A. Pharmacological studies of anthralin erythema. Br J Dermatol. 1981 Aug;105 (Suppl 20):82–86. doi: 10.1111/j.1365-2133.1981.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Norris D. A. Cytokine modulation of adhesion molecules in the regulation of immunologic cytotoxicity of epidermal targets. J Invest Dermatol. 1990 Dec;95(6 Suppl):111S–120S. doi: 10.1111/1523-1747.ep12874977. [DOI] [PubMed] [Google Scholar]
- Norris P., Poston R. N., Thomas D. S., Thornhill M., Hawk J., Haskard D. O. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991 May;96(5):763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Labhardt A. M., Sinigaglia F. Selective interaction of Ni with an MHC-bound peptide. EMBO J. 1991 Jun;10(6):1303–1306. doi: 10.1002/j.1460-2075.1991.tb07648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sterry W., Künne N., Weber-Matthiesen K., Brasch J., Mielke V. Cell trafficking in positive and negative patch-test reactions: demonstration of a stereotypic migration pathway. J Invest Dermatol. 1991 Apr;96(4):459–462. doi: 10.1111/1523-1747.ep12470141. [DOI] [PubMed] [Google Scholar]