Abstract
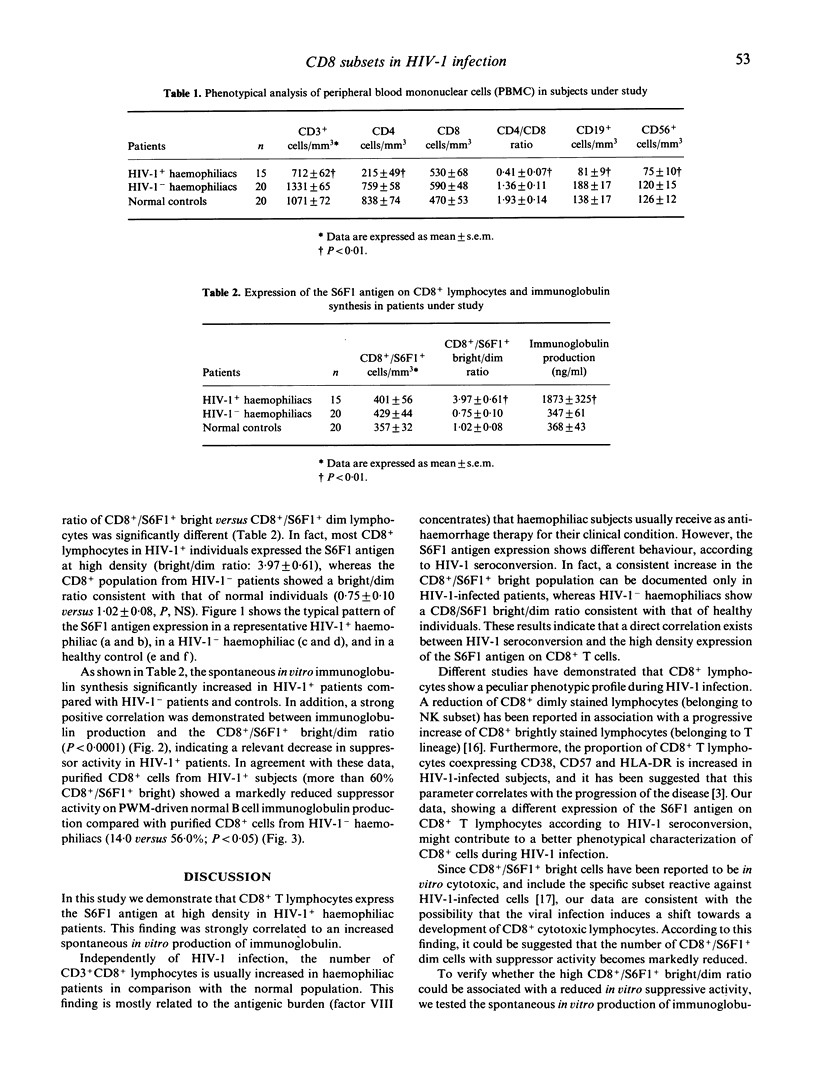
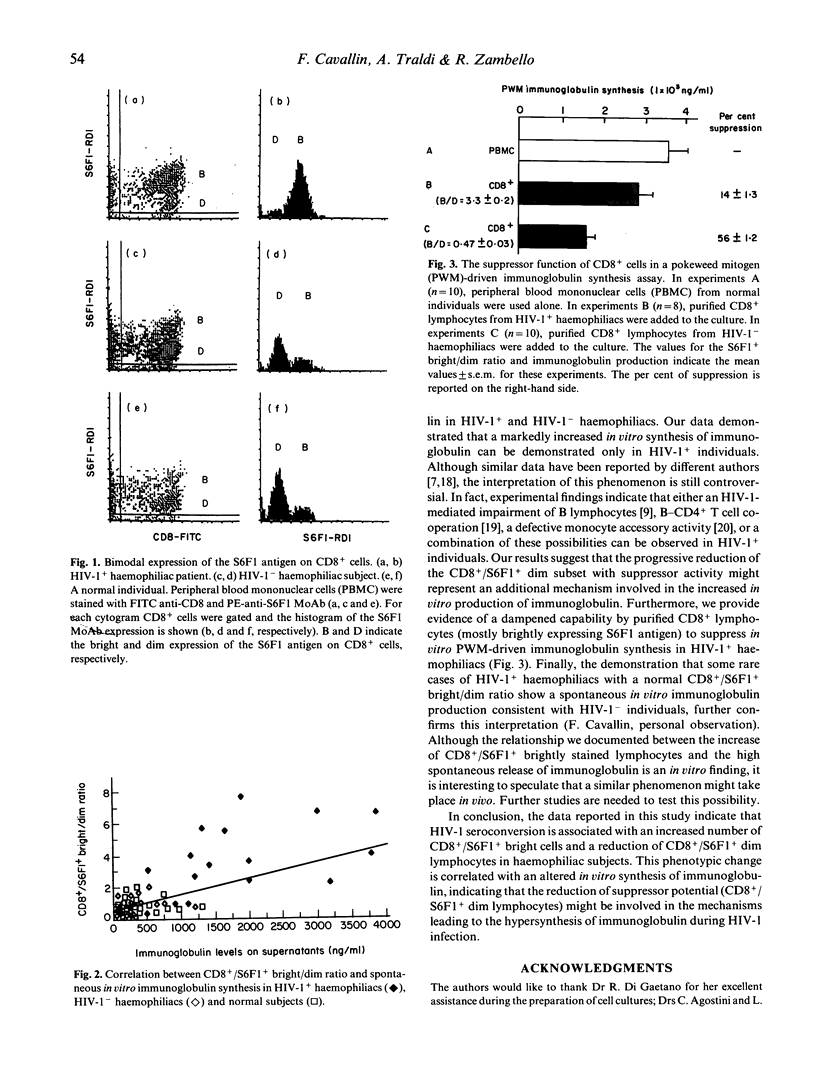
In this study we investigated the distribution of the S6F1 antigen, an epitope of the lymphocyte function-associated antigen, on CD8+ T lymphocytes in a series of 15 HIV-1+ and 20 HIV-1- haemophiliac patients. MoAbs recognizing the S6F1 antigen have been claimed to distinguish between killer effectors (brightly S6F1+ stained) and suppressor cells (dimly S6F1+ stained) within the CD8+ lymphoid population. In addition, we tried to find a correlation between the spontaneous in vitro immunoglobulin synthesis from patients' peripheral blood lymphocytes and the pattern of S6F1 expression. Although the total number of double-positive CD8+/S6F1+ cells was similar in both HIV-1+ and HIV-1- haemophiliac patients, a significant increase in the CD8+/S6F1+ population bright versus dim was documented in HIV-1-infected with respect to HIV-1- haemophiliacs (bright/dim ratio 3.97 +/- 0.61 versus 0.75 +/- 0.1, respectively, P < 0.005). This finding was correlated to a significant increase in spontaneous in vitro immunoglobulin production in HIV-1+ subjects compared with control haemophiliacs (P < 0.005). Purified CD8+ lymphocytes from HIV-1+ subjects showed a reduced suppressor activity on mitogen-induced immunoglobulin production. Taken together, these data suggest that HIV-1 infection favours the generation of CD8+/S6F1+ bright cells with putative cytotoxic-associated function, leading to a progressive reduction in the number of CD8+/S6F1+ dim suppressor lymphocytes. This phenomenon may contribute to the polyclonal hypergammaglobulinaemia present in HIV-1+ haemophiliac patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadori A., Zamarchi R., Ciminale V., Del Mistro A., Siervo S., Alberti A., Colombatti M., Chieco-Bianchi L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J Immunol. 1989 Oct 1;143(7):2146–2152. [PubMed] [Google Scholar]
- Edelman A. S., Zolla-Pazner S. Response of mononuclear cells from HIV-infected patients to B-cell mitogens: correlation with immunological and clinical features of disease progression. AIDS. 1990 Sep;4(9):859–864. doi: 10.1097/00002030-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989 Jul;52(1):10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Katz I. R., Krown S. E., Safai B., Oettgen H. F., Hoffmann M. K. Antigen-specific and polyclonal B-cell responses in patients with acquired immunodeficiency disease syndrome. Clin Immunol Immunopathol. 1986 Jun;39(3):359–367. doi: 10.1016/0090-1229(86)90164-9. [DOI] [PubMed] [Google Scholar]
- Kreiss J. K., Kitchen L. W., Prince H. E., Kasper C. K., Goldstein A. L., Naylor P. H., Preble O., Stewart J. A., Essex M. Human T cell leukemia virus type III antibody, lymphadenopathy, and acquired immune deficiency syndrome in hemophiliac subjects. Results of a prospective study. Am J Med. 1986 Mar;80(3):345–350. doi: 10.1016/0002-9343(86)90704-7. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Macchia D., Parronchi P., Piccinni M. P., Simonelli C., Mazzetti M., Ravina A., Milo D., Maggi E., Romagnani S. In vitro infection with HIV enables human CD4+ T cell clones to induce noncognate contact-dependent polyclonal B cell activation. J Immunol. 1991 May 15;146(10):3413–3418. [PubMed] [Google Scholar]
- Madhok R., Gracie J. A., Forbes C. D., Lowe G. D. B cell dysfunction in haemophilia in the absence and presence of HIV-1 infection. Thromb Haemost. 1991 Jan 23;65(1):7–10. [PubMed] [Google Scholar]
- Mansour I., Doinel C., Rouger P. Dichotomy of two CD8+ lymphocyte subsets in HIV infection. Depletion of CD8+ CD3- and expansion of CD8+ CD3+ subsets: consequence on the CD4/CD8 ratio. Clin Exp Immunol. 1991 Sep;85(3):481–484. doi: 10.1111/j.1365-2249.1991.tb05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema F., Petit A. J., Terpstra F. G., Schattenkerk J. K., de Wolf F., Al B. J., Roos M., Lange J. M., Danner S. A., Goudsmit J. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988 Dec;82(6):1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuma H., Litwin S., Zolla-Pazner S. B-cell activation in HIV infection: relationship of spontaneous immunoglobulin secretion to various immunological parameters. Clin Exp Immunol. 1988 Mar;71(3):410–416. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Rudd C. E., Letvin N. L., Schlossman S. F. A novel epitope of the LFA-1 antigen which can distinguish killer effector and suppressor cells in human CD8 cells. Nature. 1987 Dec 3;330(6147):479–482. doi: 10.1038/330479a0. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Martínez-Maza O., Hirano T., Breen E. C., Nishanian P. G., Salazar-Gonzalez J. F., Fahey J. L., Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989 Jan 15;142(2):531–536. [PubMed] [Google Scholar]
- Raska K., Jr, Kim H. C., Raska K., 3rd, Martin E., Raskova J., Saidi P. Human immunodeficiency virus (HIV) infection in haemophiliacs: long-term prognostic significance of the HIV serologic pattern. Clin Exp Immunol. 1989 Jul;77(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., Tramont E. C. The Walter Reed staging classification for HTLV-III/LAV infection. N Engl J Med. 1986 Jan 9;314(2):131–132. doi: 10.1056/NEJM198601093140232. [DOI] [PubMed] [Google Scholar]
- Sjamsoedin-Visser E. J., Heijnen C. J., Zegers B. J., Stoop J. W. Defect in B cell function in HTLV III/LAV positive hemophilia patients. Blood. 1987 May;69(5):1388–1393. [PubMed] [Google Scholar]
- Terpstra F. G., Al B. J., Roos M. T., De Wolf F., Goudsmit J., Schellekens P. T., Miedema F. Longitudinal study of leukocyte functions in homosexual men seroconverted for HIV: rapid and persistent loss of B cell function after HIV infection. Eur J Immunol. 1989 Apr;19(4):667–673. doi: 10.1002/eji.1830190415. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambello R., Trentin L., Bulian P., Cassatella M., Raimondi R., Chisesi T., Agostini C., Semenzato G. Role of tumor necrosis factor-alpha and its specific 55-Kd and 75-Kd receptors in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1992 Oct 15;80(8):2030–2037. [PubMed] [Google Scholar]


