Abstract
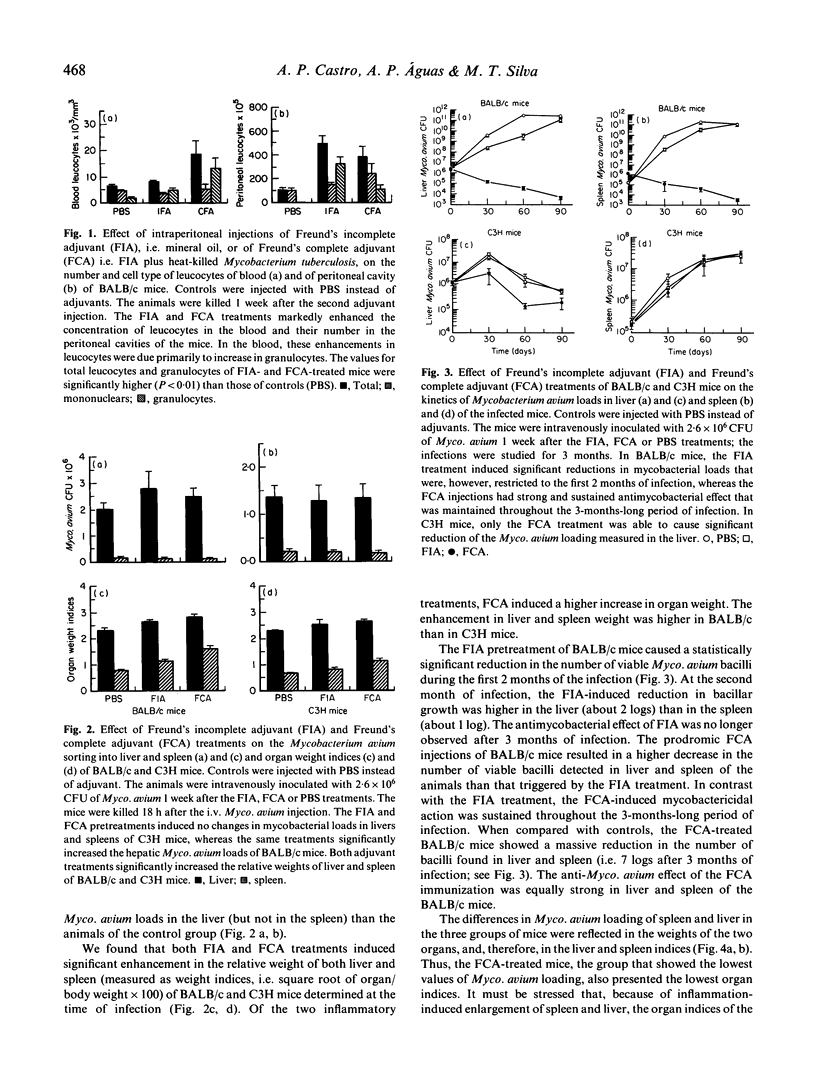
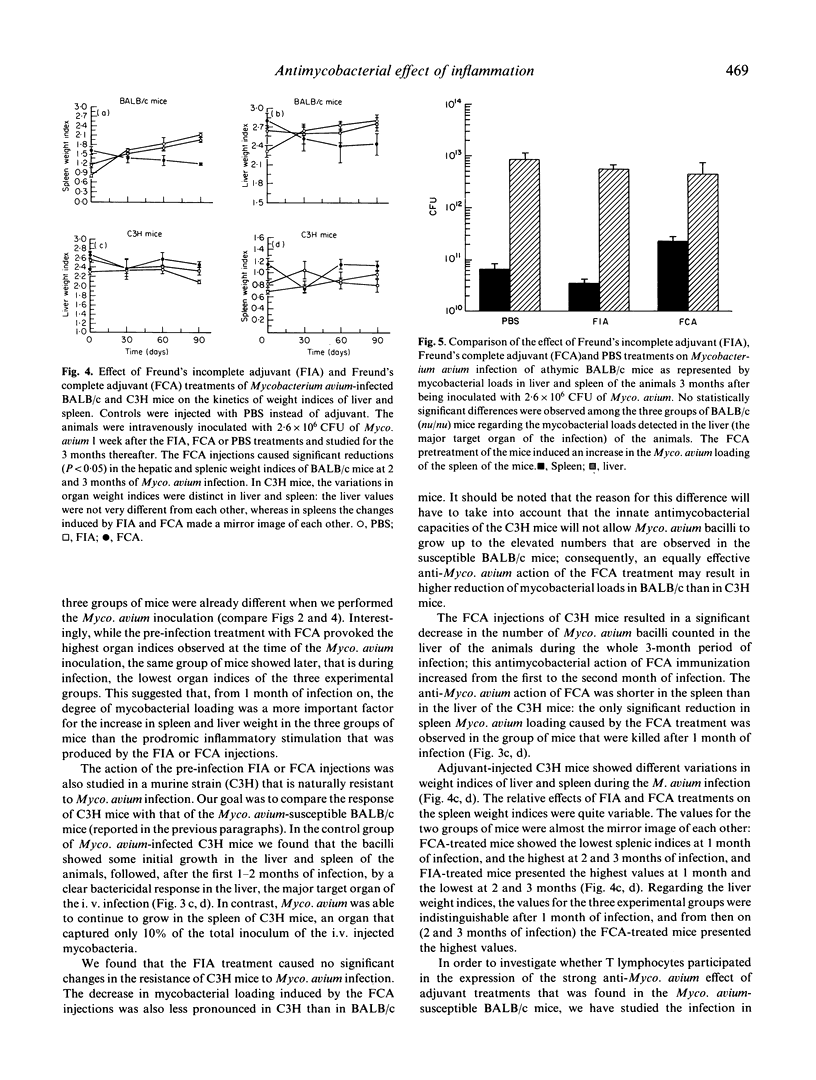
We have investigated the effect of inflammation on host resistance against infection by Mycobacterium avium, an atypical mycobacteria species that is responsible for life-threatening opportunistic infections in AIDS patients. Inflammation was induced in BALB/c mice by two intraperitoneal injections of mineral oil (Freund's incomplete adjuvant, FIA). The BALB/c strain was chosen because it is naturally susceptible to Myco. avium infection. One week after the second FIA injection, the BALB/c mice were infected intravenously with 2.6 x 10(6) Myco. avium bacilli; at this time, the mice showed systemic granulocytosis because of the FIA injections. The kinetics of the murine infection was determined during 3 months by quantification of Myco. avium loads in the major target organs (liver and spleen) of the mycobacteria. The FIA treatment resulted in a significant decrease in the growth of Myco. avium in the infected BALB/c mice. This enhancement in host resistance to Myco. avium infection lasted for 2-3 months. In contrast with BALB/c animals, C3H mice (naturally resistant to Myco. avium infection) did not show an increased anti-Myco. avium action in association with the FIA treatment. The antimycobacterial effect of the FIA injections in BALB/c mice was compared with that produced by the injection of mycobacterial antigens (heat-killed Myco. tuberculosis) added to the mineral oil (i.e. Freund's complete adjuvant, FCA). The FCA treatment resulted in strong and sustained enhancements in the microbicidal capacities of BALB/c, and also of C3H mice. Data obtained with mutant athymic BALB/c mice revealed that the anti-Myco. avium effect of the FCA treatment was T cell-dependent. Our results indicate that: (i) non-immune inflammatory stimulation (FIA) of Myco. avium-susceptible hosts is able to cause a significant, albeit transient, increase in the resistance to Myco. avium infection; (ii) this protective effect is enhanced if heat-killed mycobacteria are added to the phlogistic agent (FCA), i.e. if a T cell-dependent response is induced; and (iii) systemic increase in the number of circulating granulocytes may help host defence against Myco. avium infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Barclay W. R., Brehmer W., Larson C. L., Ribi E. Duration of immunity to tuberculosis in mice vaccinated intravenously with oil-treated cell walls of Mycobacterium bovis strain BCG. J Immunol. 1967 Jun;98(6):1265–1273. [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. M., Silva M. T. Host and bacterial factors control the Mycobacterium avium-induced chronic peritoneal granulocytosis in mice. Clin Exp Immunol. 1991 Feb;83(2):231–236. doi: 10.1111/j.1365-2249.1991.tb05620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Silva M. T. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989 Dec;78(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- Barclay W. R., Anacker R., Brehmer W., Ribi E. Effects of oil-treated mycobacterial cell walls on the organs of mice. J Bacteriol. 1967 Nov;94(5):1736–1745. doi: 10.1128/jb.94.5.1736-1745.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. I. Effect on the antibody response to bovine serum albumin and sheep red blood cells of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):426–434. [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Holzer T. J., Andersen B. R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987 Dec;156(6):985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- Castro A. G., Esaguy N., Macedo P. M., Aguas A. P., Silva M. T. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect Immun. 1991 Sep;59(9):3009–3014. doi: 10.1128/iai.59.9.3009-3014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989 Oct;2(4):360–377. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M. A., Cheung O. T., Sanders D. E., Goldstein R. S. Pulmonary infection with nontuberculous mycobacteria. Am Rev Respir Dis. 1988 Jan;137(1):149–152. doi: 10.1164/ajrccm/137.1.149. [DOI] [PubMed] [Google Scholar]
- Denis M. In vivo modulation of atypical mycobacterial infection: adjuvant therapy increases resistance to Mycobacterium avium by enhancing macrophage effector functions. Cell Immunol. 1991 Apr 15;134(1):42–53. doi: 10.1016/0008-8749(91)90329-a. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Ellis M., Gupta S., Galant S., Hakim S., VandeVen C., Toy C., Cairo M. S. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. J Infect Dis. 1988 Dec;158(6):1268–1276. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- Fontan E., Fauve R. M. Increased phagocytic activity in mice treated by a mouse granuloma protein. Ann Inst Pasteur Immunol. 1986 Jul-Aug;137D(1):93–107. [PubMed] [Google Scholar]
- Geertsma M. F., Nibbering P. H., Pos O., Van Furth R. Interferon-gamma-activated human granulocytes kill ingested Mycobacterium fortuitum more efficiently than normal granulocytes. Eur J Immunol. 1990 Apr;20(4):869–873. doi: 10.1002/eji.1830200423. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Herbert W. J. The mode of action of mineral-oil emulsion adjuvants on antibody production in mice. Immunology. 1968 Mar;14(3):301–318. [PMC free article] [PubMed] [Google Scholar]
- Jones G. S., Amirault H. J., Andersen B. R. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990 Sep;162(3):700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- Meyer T. J., Ribi E., Azuma I. Biologically active components from mycobacterial cell walls. V. Granuloma formation in mouse lungs and guinea pig skin. Cell Immunol. 1975 Mar;16(1):11–24. doi: 10.1016/0008-8749(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Michel J. C., Hurtrel B., Lagrange P. H. Incidence d'une réaction inflammatoire sur la résistance des souris envers Plasmodium berghei. Ann Immunol (Paris) 1982 Jan-Feb;133C(1):97–101. [PubMed] [Google Scholar]
- Modilevsky T., Sattler F. R., Barnes P. F. Mycobacterial disease in patients with human immunodeficiency virus infection. Arch Intern Med. 1989 Oct;149(10):2201–2205. [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The immune response to the cell wall of Mycobacterium bovis BCG. Clin Exp Immunol. 1988 Mar;71(3):388–393. [PMC free article] [PubMed] [Google Scholar]
- Prince D. S., Peterson D. D., Steiner R. M., Gottlieb J. E., Scott R., Israel H. L., Figueroa W. G., Fish J. E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989 Sep 28;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- Ribi E., Larson C., Wicht W., List R., Goode G. Effective nonliving vaccine against experimental tuberculosis in mice. J Bacteriol. 1966 Mar;91(3):975–983. doi: 10.1128/jb.91.3.975-983.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Appelberg R., Silva M. N., Macedo P. M. In vivo killing and degradation of Mycobacterium aurum within mouse peritoneal macrophages. Infect Immun. 1987 Sep;55(9):2006–2016. doi: 10.1128/iai.55.9.2006-2016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Skamene E. Genetic control of susceptibility to mycobacterial infections. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S394–S399. doi: 10.1093/clinids/11.supplement_2.s394. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A. Y., Denner J. C., Brennan P. J., McClatchy J. K. Clinical and epidemiological importance of typing of Mycobacterium avium complex isolates. J Clin Microbiol. 1992 Feb;30(2):479–484. doi: 10.1128/jcm.30.2.479-484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Vogel F. R., Chedid L. A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- Woodard L. F. Surface chemistry and classification of vaccine adjuvants and vehicles. Adv Biotechnol Processes. 1990;13:281–306. [PubMed] [Google Scholar]


