Abstract
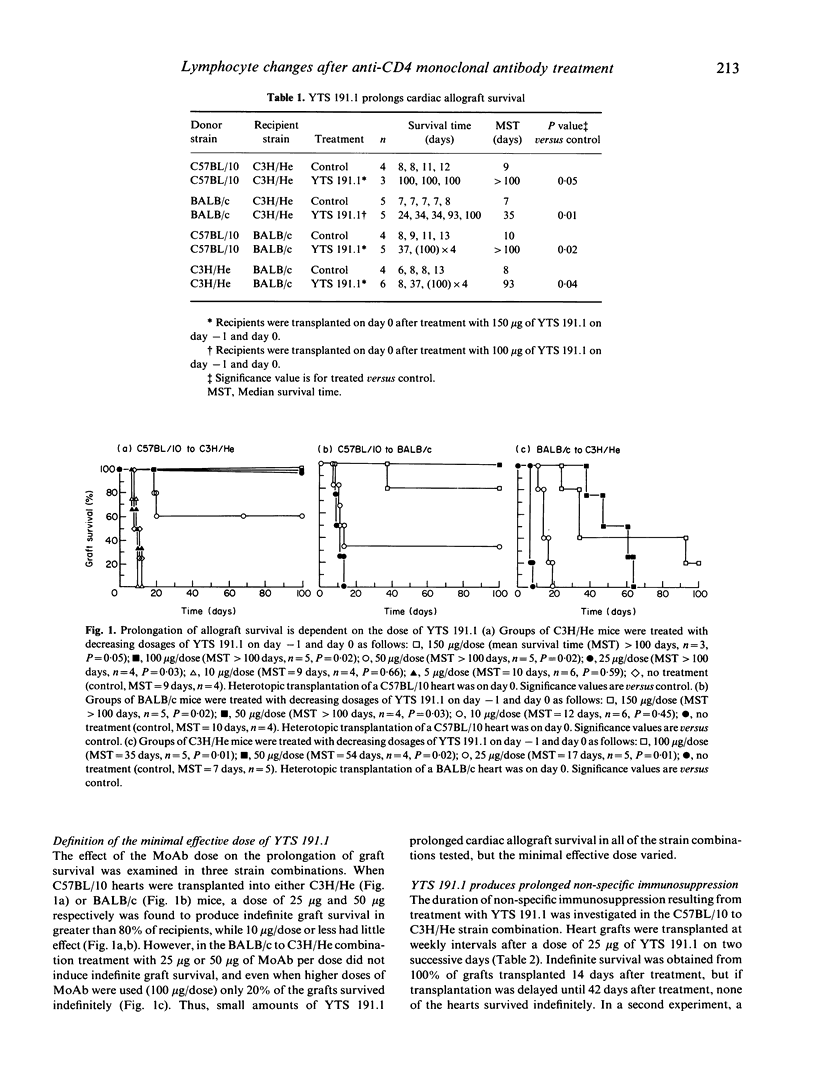
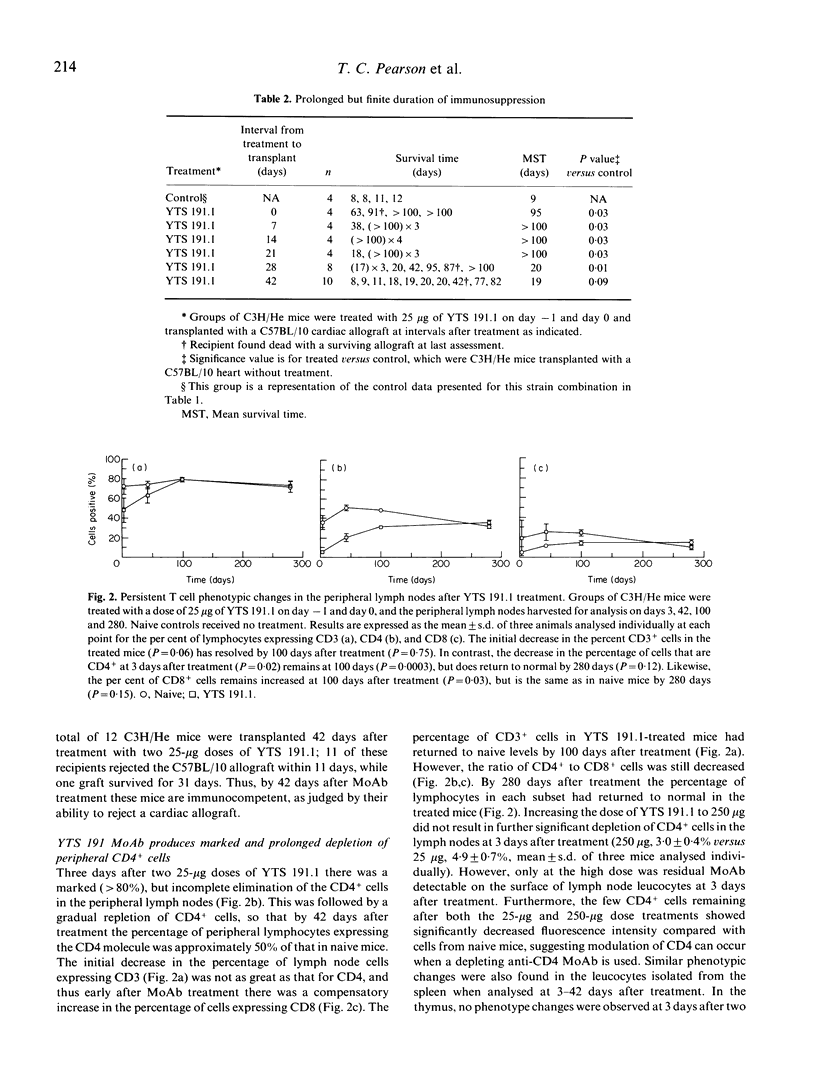
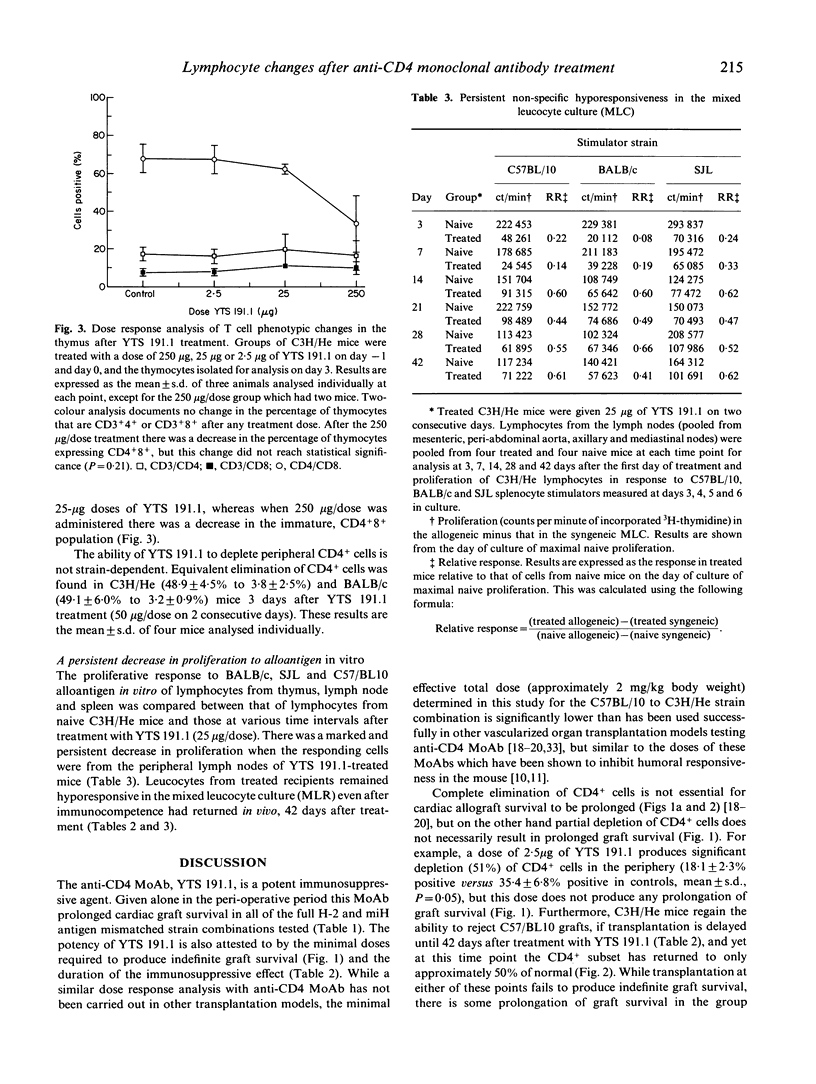
This study investigated the effect of anti-CD4 MoAb treatment on lymphocyte phenotype and function and correlated these changes with the prolongation of cardiac allograft survival in adult mice. Indefinite survival of heterotopic cardiac allografts was obtained in several fully allogeneic strain combinations when two doses of the anti-CD4 MoAb, YTS 191.1, were given at the time of transplantation. A dose response analysis in the C57BL/10 to C3H/He strain combination showed that very low doses of YTS 191.1 (25 micrograms/dose) were able to induce prolonged allograft survival when administered perioperatively. At the time of transplantation the immunosuppression induced by administration of the anti-CD4 MoAb is not antigen-specific, as heart grafts from different donor strains, mismatched for both major and minor histocompatibility antigens, showed prolonged survival in treated recipients. Immunocompetence was restored by 6 weeks after MoAb treatment, as recipients regained the ability to reject a cardiac allograft transplanted at this time point. However, while recovery of immunocompetence could be demonstrated in vivo, leucocytes isolated from the peripheral lymphoid organs of treated mice continued to be hyporesponsive in mixed leucocyte culture (MLC). Phenotypic analysis of the peripheral lymphoid tissues showed that C3H/He recipients treated with 25 micrograms/dose of YTS 191.1 had a marked, but not complete, elimination of the CD4+ subset at the time of transplantation, which was gradually restored to 50% of normal by 6 weeks after treatment. Thus, complete elimination of the CD4+ subset was not required to achieve indefinite allograft survival, and immunocompetence, as assessed in vivo, returned even when the CD4+ subset was present at half the normal level. Low doses of anti-CD4 MoAb (25 micrograms) had no effect on the expression of the CD4 molecule by thymocytes, and yet thymocytes were hyporesponsive to alloantigen in vitro. At higher doses of YTS 191.1, immature CD4+8+ thymocytes were selectively depleted. These results suggest that anti-CD4 MoAb therapy may modulate the intrathymic T cell selection process. These studies provide further insight into the mechanism of action of low dose, depleting anti-CD4 MoAb therapy in allograft rejection, and form a basis from which rational modifications to therapeutic protocols in transplantation models can be made.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr, Ghobrial R. R., Russell P. S., Winn H. J. Prevention of alloantibody formation after skin grafting without prolongation of graft survival by anti-L3T4 in vivo. Transplantation. 1988 Jun;45(6):1118–1123. doi: 10.1097/00007890-198806000-00024. [DOI] [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton E. M., Gracie J. A., Briggs J. D., Kampinga J., Bradley J. A. Cellular requirements for renal allograft rejection in the athymic nude rat. J Exp Med. 1989 Jun 1;169(6):1931–1946. doi: 10.1084/jem.169.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Martin G., Waldmann H. The induction of skin graft tolerance in major histocompatibility complex-mismatched or primed recipients: primed T cells can be tolerized in the periphery with anti-CD4 and anti-CD8 antibodies. Eur J Immunol. 1990 Dec;20(12):2747–2755. doi: 10.1002/eji.1830201232. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Waldmann H. Skin allograft rejection by L3/T4+ and Lyt-2+ T cell subsets. Transplantation. 1986 May;41(5):634–639. doi: 10.1097/00007890-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Delmonico F. L., Wright J. K., Wee S. L., Preffer F. I., Bedle M., Colvin R. B. OKT4A monoclonal antibody immunosuppression of cynomolgus renal allograft recipients. Transplant Proc. 1991 Feb;23(1 Pt 1):501–503. [PubMed] [Google Scholar]
- Coulie P. G., Coutelier J. P., Uyttenhove C., Lambotte P., Van Snick J. In vivo suppression of T-dependent antibody responses by treatment with a monoclonal anti-L3T4 antibody. Eur J Immunol. 1985 Jun;15(6):638–640. doi: 10.1002/eji.1830150620. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W. Role of thymus-derived and thymus-independent cells in murine skin allograft rejection. Transplantation. 1982 Mar;33(3):221–223. doi: 10.1097/00007890-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J., Roser B. Lymphocyte subpopulations and memory of MHC antigens. I. Quantitative aspects of neonatal heart graft rejection in normal and immune rats. Transplantation. 1987 Apr;43(4):556–560. doi: 10.1097/00007890-198704000-00020. [DOI] [PubMed] [Google Scholar]
- Herbert J., Roser B. Strategies of monoclonal antibody therapy that induce permanent tolerance of organ transplants. Transplantation. 1988 Aug;46(2 Suppl):128S–134S. doi: 10.1097/00007890-198808001-00024. [DOI] [PubMed] [Google Scholar]
- Herzog C., Walker C., Pichler W., Aeschlimann A., Wassmer P., Stockinger H., Knapp W., Rieber P., Müller W. Monoclonal anti-CD4 in arthritis. Lancet. 1987 Dec 19;2(8573):1461–1462. doi: 10.1016/s0140-6736(87)91158-5. [DOI] [PubMed] [Google Scholar]
- Ilano A. L., McConnell M. V., Gurley K. E., Spinelli A., Pearce N. W., Hall B. M. Cellular basis of allograft rejection in vivo. V. Examination of the mechanisms responsible for the differing efficacy of monoclonal antibody to CD4+ T cell subsets in low- and high-responder rat strains. J Immunol. 1989 Nov 1;143(9):2828–2836. [PubMed] [Google Scholar]
- Jonker M., Neuhaus P., Zurcher C., Fucello A., Goldstein G. OKT4 and OKT4A antibody treatment as immunosuppression for kidney transplantation in rhesus monkeys. Transplantation. 1985 Mar;39(3):247–253. doi: 10.1097/00007890-198503000-00006. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., McKenzie I. F. Which T cells cause graft rejection? Transplantation. 1982 Mar;33(3):217–221. doi: 10.1097/00007890-198203000-00001. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- Madsen J. C., Peugh W. N., Wood K. J., Morris P. J. The effect of anti-L3T4 monoclonal antibody treatment on first-set rejection of murine cardiac allografts. Transplantation. 1987 Dec;44(6):849–852. [PubMed] [Google Scholar]
- Mathieson P. W., Cobbold S. P., Hale G., Clark M. R., Oliveira D. B., Lockwood C. M., Waldmann H. Monoclonal-antibody therapy in systemic vasculitis. N Engl J Med. 1990 Jul 26;323(4):250–254. doi: 10.1056/NEJM199007263230407. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Kruisbeek A. M., Uppenkamp I. K., Sharrow S. O., Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988 Nov 3;336(6194):76–79. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- Morris P. J. Clinical highlights of the 1990 Congress. Transplant Proc. 1991 Feb;23(1 Pt 1):25–27. [PubMed] [Google Scholar]
- Mottram P. L., Wheelahan J., McKenzie I. F., Clunie G. J. Murine cardiac allograft survival following treatment of recipients with monoclonal anti-L3T4 or Ly-2 antibodies. Transplant Proc. 1987 Apr;19(2):2898–2901. [PubMed] [Google Scholar]
- Nieuwenhuis P., Stet R. J., Wagenaar J. P., Wubbena A. S., Kampinga J., Karrenbeld A. The transcapsular route: a new way for (self-) antigens to by-pass the blood-thymus barrier? Immunol Today. 1988 Dec;9(12):372–375. doi: 10.1016/0167-5699(88)91236-4. [DOI] [PubMed] [Google Scholar]
- Qin S., Cobbold S., Tighe H., Benjamin R., Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987 Aug;17(8):1159–1165. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- Raviola E., Karnovsky M. J. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972 Sep 1;136(3):466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh M. H., Sablinski T., Tanaka K., Kut J. P., Kwok C. A., Tilney N. L., Kupiec-Weglinski J. W., Milford E. L. Effects of BWH-4 anti-CD4 monoclonal antibody on rat vascularized cardiac allografts before and after engraftment. Transplantation. 1991 Feb;51(2):296–299. doi: 10.1097/00007890-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Gregory A. K., Chao C. T., Fathman C. G. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987 Jul 17;237(4812):278–280. doi: 10.1126/science.2955518. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Seydel K. B., Flavin T. F., Wu A. P., Kong C. C., Hoyt E. G., Fujimoto N., Billingham M. E., Starnes V. A., Fathman C. G. Induction of donor-specific unresponsiveness to cardiac allografts in rats by pretransplant anti-CD4 monoclonal antibody therapy. Transplantation. 1990 Sep;50(3):366–373. doi: 10.1097/00007890-199009000-00002. [DOI] [PubMed] [Google Scholar]
- Soulillou J. P., Cantarovich D., Le Mauff B., Giral M., Robillard N., Hourmant M., Hirn M., Jacques Y. Randomized controlled trial of a monoclonal antibody against the interleukin-2 receptor (33B3.1) as compared with rabbit antithymocyte globulin for prophylaxis against rejection of renal allografts. N Engl J Med. 1990 Apr 26;322(17):1175–1182. doi: 10.1056/NEJM199004263221702. [DOI] [PubMed] [Google Scholar]
- Steinmuller D., Snider M. E., Noble R. L., Waldschmidt T. J. Dissociation of tissue destruction induced by cytolytic T cells in vivo and cytotoxicity as measured in vitro. Transplantation. 1990 Oct;50(4):663–668. doi: 10.1097/00007890-199010000-00027. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Strome P., Wood P. J. Failure of in vitro assays to predict accurately the existence of neonatally induced H-2 tolerance. Transplantation. 1989 Oct;48(4):630–634. [PubMed] [Google Scholar]
- Superina R. A., Peugh W. N., Wood K. J., Morris P. J. Assessment of primarily vascularized cardiac allografts in mice. Transplantation. 1986 Aug;42(2):226–227. [PubMed] [Google Scholar]
- Teh H. S., Garvin A. M., Forbush K. A., Carlow D. A., Davis C. B., Littman D. R., Perlmutter R. M. Participation of CD4 coreceptor molecules in T-cell repertoire selection. Nature. 1991 Jan 17;349(6306):241–243. doi: 10.1038/349241a0. [DOI] [PubMed] [Google Scholar]
- Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 1988;28(6):455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- Waldmann H. Manipulation of T-cell responses with monoclonal antibodies. Annu Rev Immunol. 1989;7:407–444. doi: 10.1146/annurev.iy.07.040189.002203. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Swarztrauber K., Fathman C. G. Immunosuppression by anti-CD4 treatment in vivo. Cellular and humoral responses to alloantigens. Transplantation. 1989 Jun;47(6):1039–1042. doi: 10.1097/00007890-198906000-00024. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Woodcock J., Wofsy D., Eriksson E., Scott J. H., Seaman W. E. Rejection of skin grafts and generation of cytotoxic T cells by mice depleted of L3T4+ cells. Transplantation. 1986 Dec;42(6):636–642. doi: 10.1097/00007890-198612000-00012. [DOI] [PubMed] [Google Scholar]


