Abstract
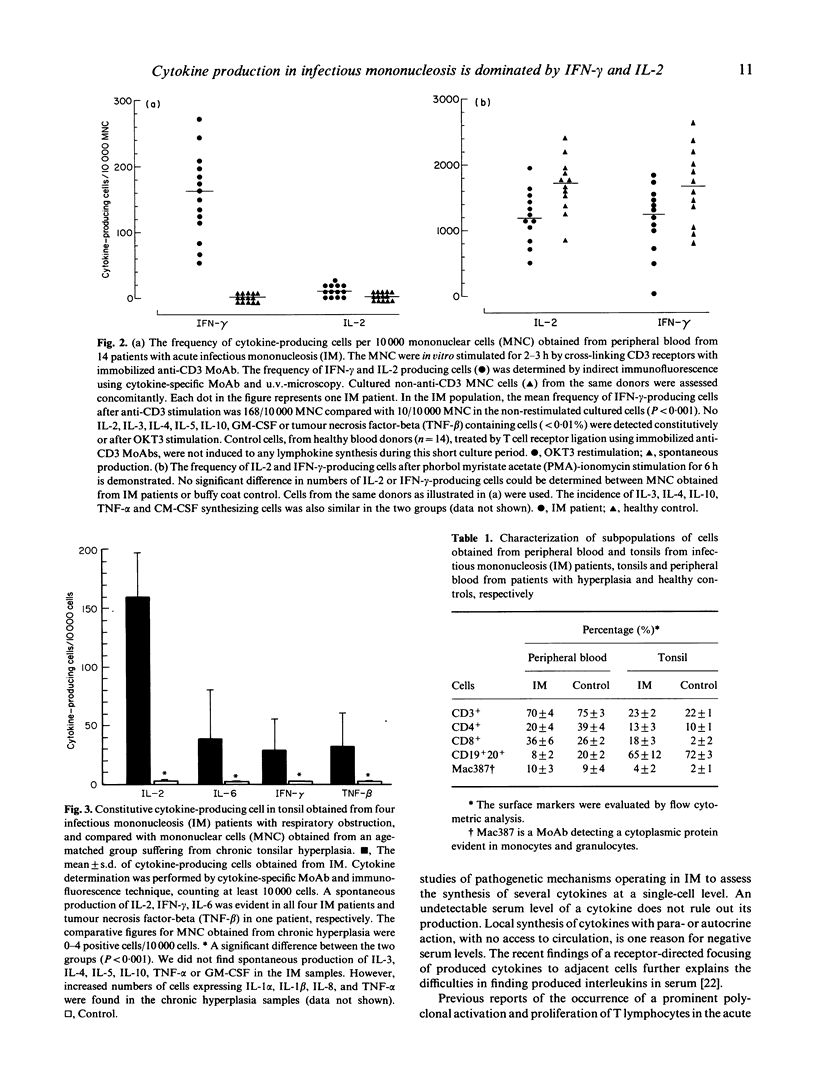
Cytokine profile and production was studied at a single-cell level in cells obtained from 14 patients with acute infectious mononucleosis (IM), with less than 7 days of symptomatic disease, by use of cytokine-specific MoAbs and indirect immunofluorescence technique. In producer cells, all the studied cytokines, except IL-1, accumulated in the Golgi system, which resulted in a characteristic morphology of the staining. Less than one in a thousand mononuclear cells obtained directly from IM blood and stained within 2 h of sampling produced IL-2, interferon-gamma (IFN-gamma), IL-4, IL-5, IL-6, IL-10, GM-CSF, tumour necrosis factor-alpha (TNF-alpha) or TNF-beta, spontaneously. However, these cells were induced to cytokine synthesis by T cell receptor ligation in vitro using immobilized anti-CD3 MoAbs for 2-3 h restimulation under conditions which did not activate normal cells. By this approach 168 +/- 120 cells/10,000 peripheral blood mononuclear cells produced IFN-gamma as compared with 10 +/- 8 cells/10,000 non-stimulated cultured cells obtained from IM patients (P < 0.001) and 1/10,000 cells obtained from healthy controls, respectively. No induced production of IL-2, IL-3, IL-4, IL-5, IL-10, GM-CSF or TNF-beta was detected in IM cells obtained from peripheral blood by this restimulation. In contrast, a spontaneous cytokine production was evident in tonsil material obtained from four IM patients tonsilectomized because of respiratory obstruction. From this site 160 +/- 40 cells/10,000 cells produced IL-2, 40 +/- 30 cells IL-6, 30 +/- 30 cells TNF-beta and 35 +/- 25 cells IFN-gamma, respectively. No such spontaneous IL-2, IL-6, TNF-beta or IFN-gamma production was evident in control cells obtained from patients tonsilectomized because of chronic tonsil hyperplasia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Andersson J., Björk L., Dinarello C. A., Towbin H., Andersson U. Lipopolysaccharide induces human interleukin-1 receptor antagonist and interleukin-1 production in the same cell. Eur J Immunol. 1992 Oct;22(10):2617–2623. doi: 10.1002/eji.1830221022. [DOI] [PubMed] [Google Scholar]
- Andersson J., Britton S., Ernberg I., Andersson U., Henle W., Sköldenberg B., Tisell A. Effect of acyclovir on infectious mononucleosis: a double-blind, placebo-controlled study. J Infect Dis. 1986 Feb;153(2):283–290. doi: 10.1093/infdis/153.2.283. [DOI] [PubMed] [Google Scholar]
- Andersson J., Nagy S., Björk L., Abrams J., Holm S., Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992 Jun;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Haworth S. J., Cohen J., Mundin J., Rickinson A., Sissons J. G. Epstein Barr virus-specific immune defects in patients with persistent symptoms following infectious mononucleosis. Q J Med. 1986 Feb;58(226):111–121. [PubMed] [Google Scholar]
- Cardell S., Sander B. Interleukin 2, 4 and 5 are sequentially produced in mitogen-stimulated murine spleen cell cultures. Eur J Immunol. 1990 Feb;20(2):389–395. doi: 10.1002/eji.1830200223. [DOI] [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Gosselin J., Flamand L., D'Addario M., Hiscott J., Menezes J. Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses. Differential induction of interleukin 6 and tumor necrosis factor-alpha. J Clin Invest. 1992 Jun;89(6):1849–1856. doi: 10.1172/JCI115789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida N., Sakurai S., Hosaka T., Hosoi K., Kunitomo T., Shimazu T., Maruyama T., Matsuura Y., Kohase M. Establishment of strongly neutralizing monoclonal antibody to human interleukin-6 and its epitope analysis. Biochem Biophys Res Commun. 1989 Dec 15;165(2):728–734. doi: 10.1016/s0006-291x(89)80027-0. [DOI] [PubMed] [Google Scholar]
- Kamio M., Uchiyama T., Hori T., Kodaka T., Ishikawa T., Onishi R., Uchino H., Yoneda N., Tatsumi E., Yamaguchi N. Selective expression of the p70 subunit of the interleukin-2 receptor on lymphocytes from patients with infectious mononucleosis. Blood. 1990 Jan 15;75(2):415–420. [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Lamche H. R., Adolf G. R. Highly sensitive enzyme immunoassays for antibodies to human tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta). J Immunol Methods. 1990 Aug 7;131(2):283–289. doi: 10.1016/0022-1759(90)90200-f. [DOI] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Dinarello C. A., Carson D. A., Vaughan J. H. Release of lymphokines after Epstein Barr virus infection in vitro. I. Sources of and kinetics of production of interferons and interleukins in normal humans. J Immunol. 1986 May 15;136(10):3636–3642. [PubMed] [Google Scholar]
- Malkovský M., Sondel P. M., Strober W., Dalgleish A. G. The interleukins in acquired disease. Clin Exp Immunol. 1988 Nov;74(2):151–161. [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E., Chen W. F., Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989 Aug 15;143(4):1210–1214. [PubMed] [Google Scholar]
- Matsuda T., Hirano T., Kishimoto T. Establishment of an interleukin 6 (IL 6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL 6 monoclonal antibodies. Eur J Immunol. 1988 Jun;18(6):951–956. doi: 10.1002/eji.1830180618. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Kasahara Y., Kanegane H., Ohta K., Yokoi T., Yachie A., Taniguchi N. Expression of CD45R0 (UCHL1) by CD4+ and CD8+ T cells as a sign of in vivo activation in infectious mononucleosis. Clin Exp Immunol. 1991 Mar;83(3):447–451. doi: 10.1111/j.1365-2249.1991.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo W. J., Conrad L., Janeway C. A., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988 Mar 24;332(6162):378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Rordorf-Adam C., Lazdins J., Woods-Cook K., Alteri E., Henn R., Geiger T., Feige U., Towbin H., Erard F. An assay for the detection of interleukin-1 synthesis inhibitors: effects of antirheumatic drugs. Drugs Exp Clin Res. 1989;15(8):355–362. [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Sander B., Cardell S., Möller E. Interleukin 4 and interferon gamma production in restimulated CD4+ and CD8+ cells indicates memory type responsiveness. Scand J Immunol. 1991 Mar;33(3):287–296. doi: 10.1111/j.1365-3083.1991.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Sheldon P. J., Hemsted E. H., Papamichail M., Holborow E. J. Thymic origin of atypical lymphoid cells in infectious mononucleosis. Lancet. 1973 May 26;1(7813):1153–1155. doi: 10.1016/s0140-6736(73)91148-3. [DOI] [PubMed] [Google Scholar]
- Shiftan T. A., Mendelsohn J. The circulating "atypical" lymphocyte. Hum Pathol. 1978 Jan;9(1):51–61. doi: 10.1016/s0046-8177(78)80007-0. [DOI] [PubMed] [Google Scholar]
- Tomkinson B. E., Wagner D. K., Nelson D. L., Sullivan J. L. Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987 Dec 1;139(11):3802–3807. [PubMed] [Google Scholar]
- Tomkinson B. E., Wagner D. K., Nelson D. L., Sullivan J. L. Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987 Dec 1;139(11):3802–3807. [PubMed] [Google Scholar]