Abstract
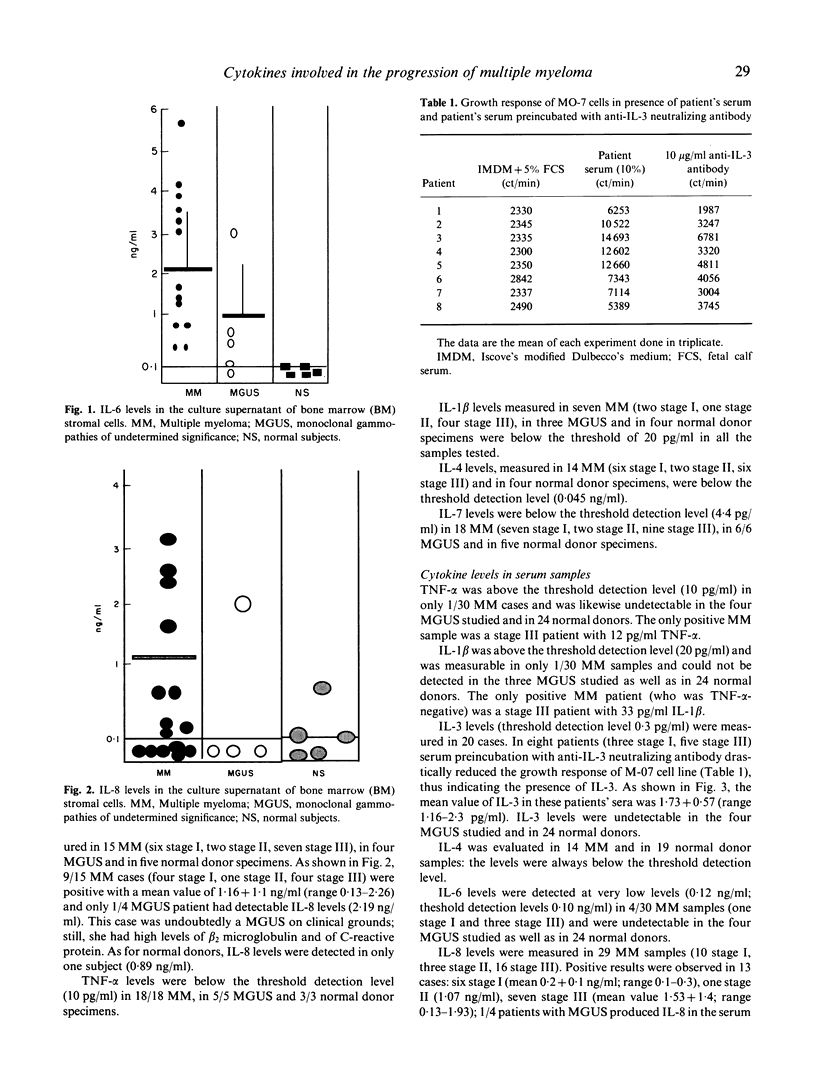
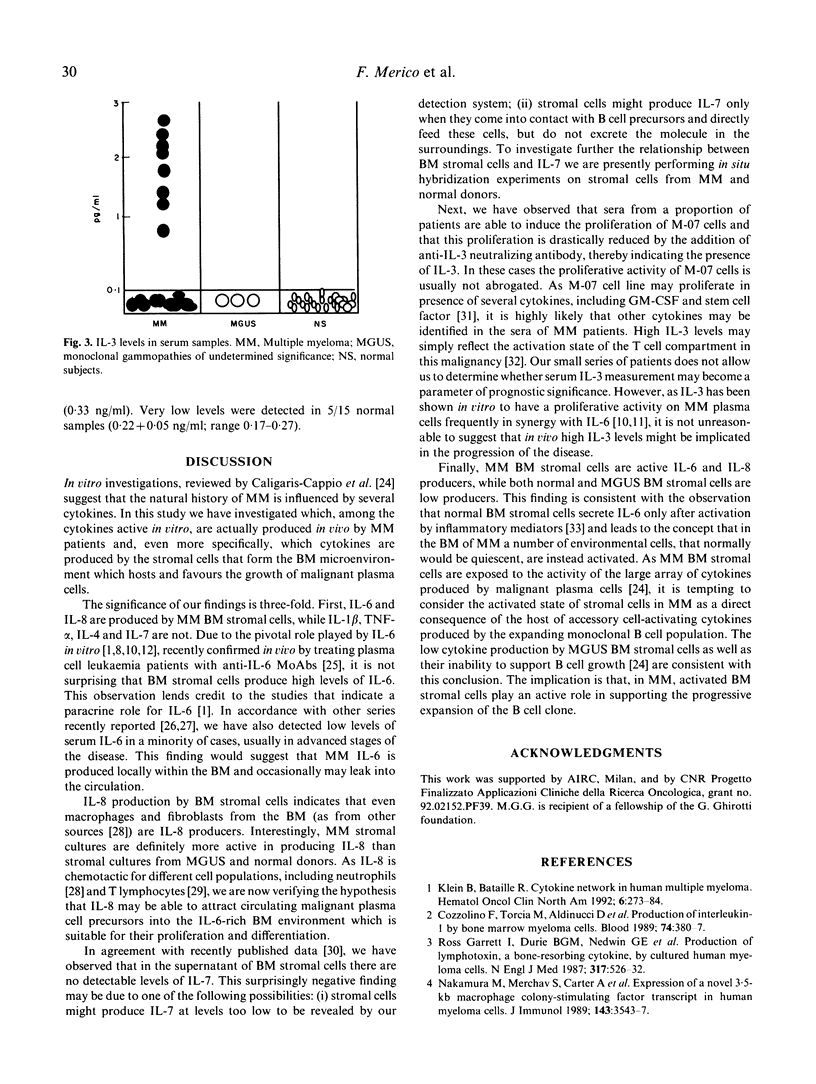
We have investigated which of the cytokines that are relevant in the in vitro growth of multiple myeloma (MM) malignant plasma cells are actually produced in vivo by MM patients. To this end, we have measured the levels of IL-1 beta, IL-3, IL-4, IL-6, IL-7, IL-8 and tumour necrosis factor-alpha (TNF-alpha) both in sera and in the supernatant of bone marrow (BM) stromal cell cultures from patients with MM and monoclonal gammopathy of undetermined significance (MGUS). The significance of our findings is three-fold. First, IL-6 and IL-8 are produced by MM BM stromal cells, while IL-1 beta, TNF-alpha, IL-4 and IL-7 are not. Second, IL-3 is the only cytokine consistently raised in serum samples: we have also detected low levels of serum IL-6 in a minority of cases, usually in advanced stage of the disease. Third, MM BM stromal cells are active IL-6 and IL-8 producers, while both normal and MGUS BM stromal cells are low producers, thus suggesting that in the BM of MM a number of environmental cells, that would normally be quiescent, are instead activated and that, in MM, activated BM stromal cells play an active role in supporting the progressive expansion of the B cell clone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. C., Jones R. M., Morimoto C., Leavitt P., Barut B. A. Response patterns of purified myeloma cells to hematopoietic growth factors. Blood. 1989 May 15;73(7):1915–1924. [PubMed] [Google Scholar]
- Avanzi G. C., Lista P., Giovinazzo B., Miniero R., Saglio G., Benetton G., Coda R., Cattoretti G., Pegoraro L. Selective growth response to IL-3 of a human leukaemic cell line with megakaryoblastic features. Br J Haematol. 1988 Jul;69(3):359–366. doi: 10.1111/j.1365-2141.1988.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Epstein J., Selvanayagam P., Alexanian R. Plasma cell myeloma--new biological insights and advances in therapy. Blood. 1989 Mar;73(4):865–879. [PubMed] [Google Scholar]
- Barton B. E., Mayer R. IL-3 induces differentiation of bone marrow precursor cells to osteoclast-like cells. J Immunol. 1989 Nov 15;143(10):3211–3216. [PubMed] [Google Scholar]
- Bataille R., Jourdan M., Zhang X. G., Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989 Dec;84(6):2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergui L., Schena M., Gaidano G., Riva M., Caligaris-Cappio F. Interleukin 3 and interleukin 6 synergistically promote the proliferation and differentiation of malignant plasma cell precursors in multiple myeloma. J Exp Med. 1989 Aug 1;170(2):613–618. doi: 10.1084/jem.170.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bergui L., Gregoretti M. G., Gaidano G., Gaboli M., Schena M., Zallone A. Z., Marchisio P. C. 'Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 1991 Jun 15;77(12):2688–2693. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gregoretti M. G., Ghia P., Bergui L. In vitro growth of human multiple myeloma: implications for biology and therapy. Hematol Oncol Clin North Am. 1992 Apr;6(2):257–271. [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Rubartelli A., Miliani A., Shaw A. R., Lansdorp P. M., Di Guglielmo R. Production of interleukin-1 by bone marrow myeloma cells. Blood. 1989 Jul;74(1):380–387. [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975 Sep;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Garrett I. R., Durie B. G., Nedwin G. E., Gillespie A., Bringman T., Sabatini M., Bertolini D. R., Mundy G. R. Production of lymphotoxin, a bone-resorbing cytokine, by cultured human myeloma cells. N Engl J Med. 1987 Aug 27;317(9):526–532. doi: 10.1056/NEJM198708273170902. [DOI] [PubMed] [Google Scholar]
- Hendrie P. C., Miyazawa K., Yang Y. C., Langefeld C. D., Broxmeyer H. E. Mast cell growth factor (c-kit ligand) enhances cytokine stimulation of proliferation of the human factor-dependent cell line, M07e. Exp Hematol. 1991 Nov;19(10):1031–1037. [PubMed] [Google Scholar]
- Jernberg H., Pettersson M., Kishimoto T., Nilsson K. Heterogeneity in response to interleukin 6 (IL-6), expression of IL-6 and IL-6 receptor mRNA in a panel of established human multiple myeloma cell lines. Leukemia. 1991 Mar;5(3):255–265. [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Klein B., Bataille R. Cytokine network in human multiple myeloma. Hematol Oncol Clin North Am. 1992 Apr;6(2):273–284. [PubMed] [Google Scholar]
- Klein B., Wijdenes J., Zhang X. G., Jourdan M., Boiron J. M., Brochier J., Liautard J., Merlin M., Clement C., Morel-Fournier B. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991 Sep 1;78(5):1198–1204. [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Content J., Houssiau F., Aarden L., Piechaczyk M., Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989 Feb;73(2):517–526. [PubMed] [Google Scholar]
- Kodama H., Nose M., Niida S., Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991 May 1;173(5):1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N., Bertolini D., Suda T., Akiyama Y., Roodman G. D. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J Immunol. 1990 Jun 1;144(11):4226–4230. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Ludwig H., Nachbaur D. M., Fritz E., Krainer M., Huber H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood. 1991 Jun 15;77(12):2794–2795. [PubMed] [Google Scholar]
- Massaia M., Bianchi A., Attisano C., Peola S., Redoglia V., Dianzani U., Pileri A. Detection of hyperreactive T cells in multiple myeloma by multivalent cross-linking of the CD3/TCR complex. Blood. 1991 Oct 1;78(7):1770–1780. [PubMed] [Google Scholar]
- Mundy G. R., Raisz L. G., Cooper R. A., Schechter G. P., Salmon S. E. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974 Nov 14;291(20):1041–1046. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Merchav S., Carter A., Ernst T. J., Demetri G. D., Furukawa Y., Anderson K., Freedman A. S., Griffin J. D. Expression of a novel 3.5-kb macrophage colony-stimulating factor transcript in human myeloma cells. J Immunol. 1989 Dec 1;143(11):3543–3547. [PubMed] [Google Scholar]
- Nemunaitis J., Andrews D. F., Mochizuki D. Y., Lilly M. B., Singer J. W. Human marrow stromal cells: response to interleukin-6 (IL-6) and control of IL-6 expression. Blood. 1989 Nov 1;74(6):1929–1935. [PubMed] [Google Scholar]
- Peichl P., Ceska M., Effenberger F., Haberhauer G., Broell H., Lindley I. J. Presence of NAP-1/IL-8 in synovial fluids indicates a possible pathogenic role in rheumatoid arthritis. Scand J Immunol. 1991 Sep;34(3):333–339. doi: 10.1111/j.1365-3083.1991.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Rooney M. L., Desjardins L. A., Heeley J. D. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987 Dec;2(6):559–565. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- Wolf M. L., Buckley J. A., Goldfarb A., Law C. L., LeBien T. W. Development of a bone marrow culture for maintenance and growth of normal human B cell precursors. J Immunol. 1991 Nov 15;147(10):3324–3330. [PubMed] [Google Scholar]
- Zhang X. G., Bataille R., Jourdan M., Saeland S., Banchereau J., Mannoni P., Klein B. Granulocyte-macrophage colony-stimulating factor synergizes with interleukin-6 in supporting the proliferation of human myeloma cells. Blood. 1990 Dec 15;76(12):2599–2605. [PubMed] [Google Scholar]
- Zhang X. G., Klein B., Bataille R. Interleukin-6 is a potent myeloma-cell growth factor in patients with aggressive multiple myeloma. Blood. 1989 Jul;74(1):11–13. [PubMed] [Google Scholar]


