Abstract
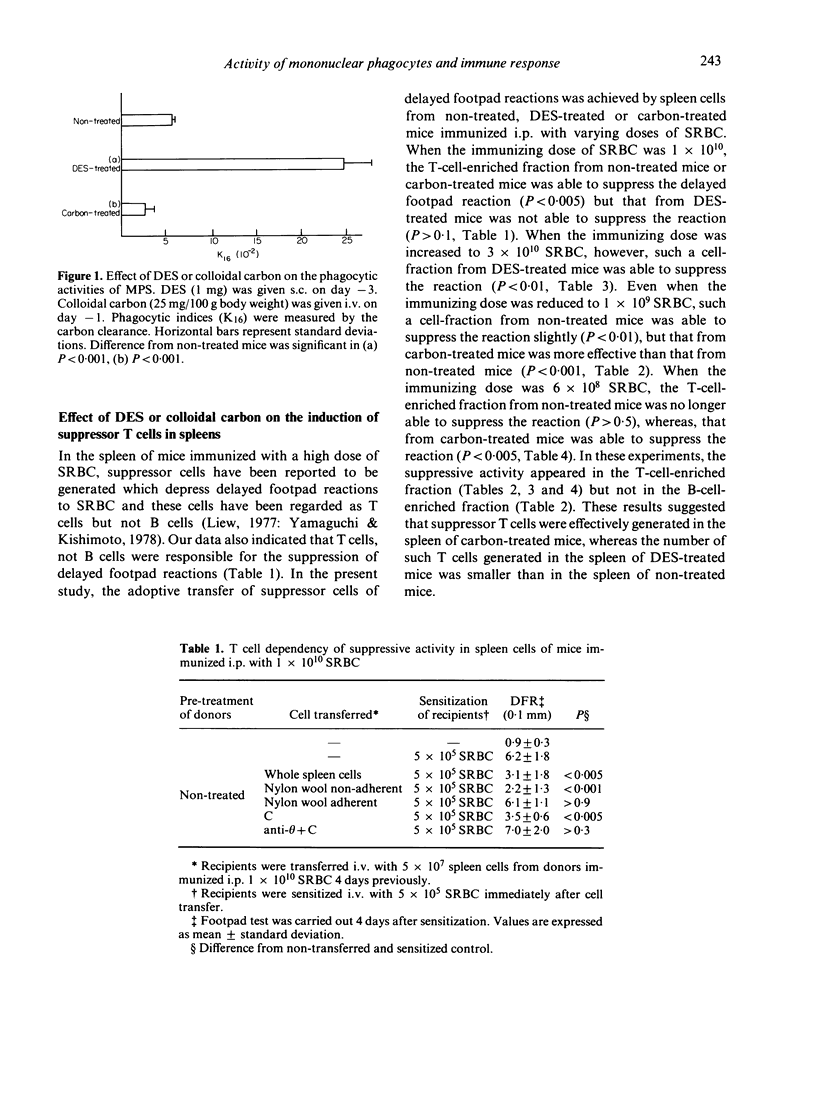
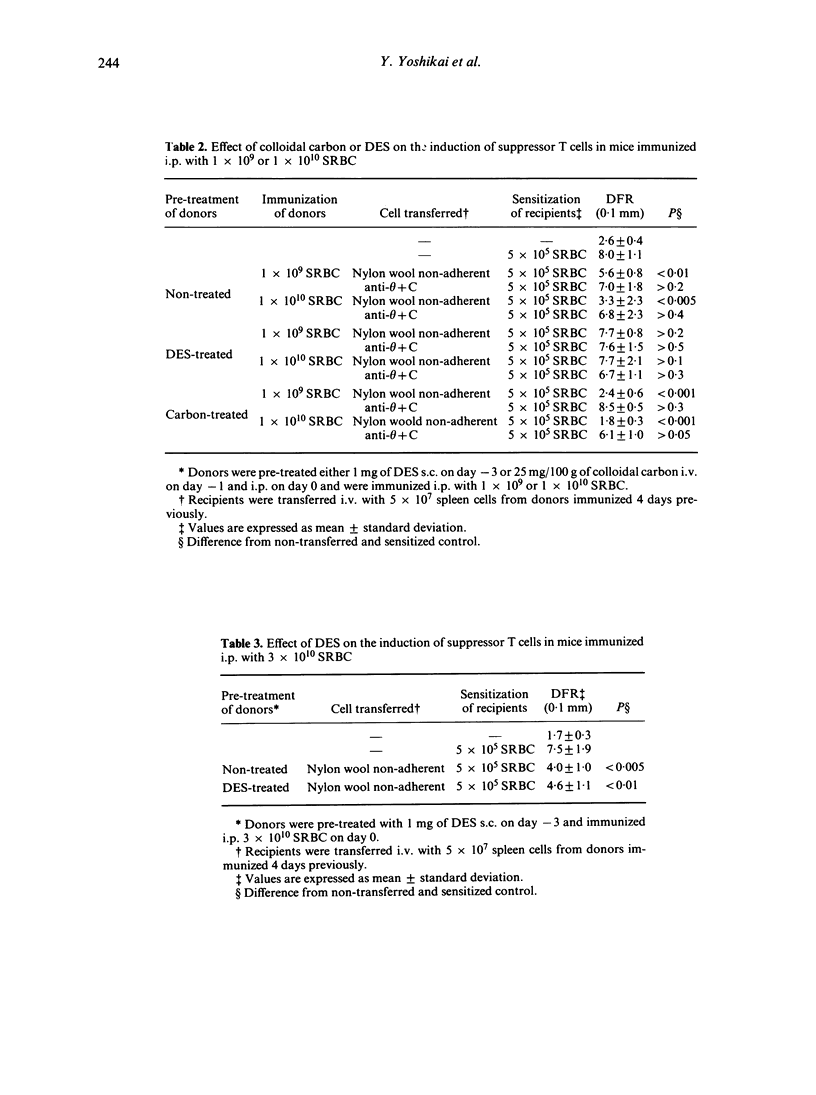
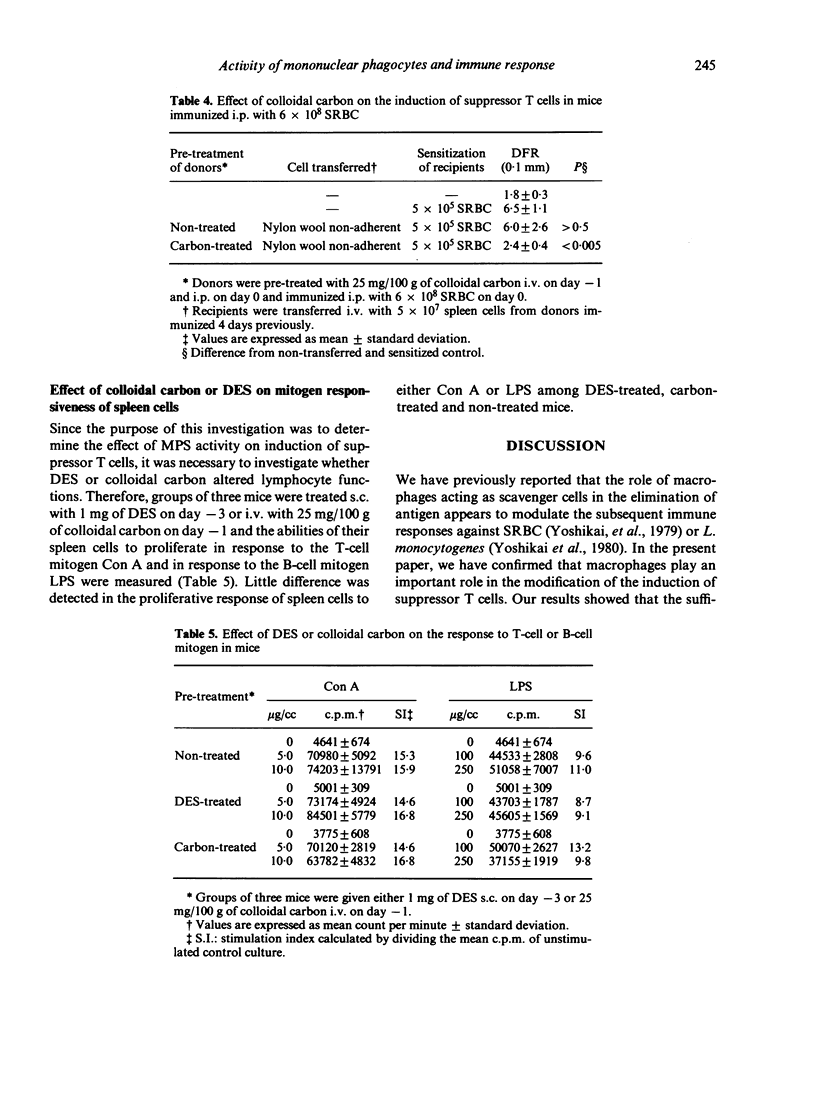
The role of mononuclear phagocyte system (MPS) in the induction of suppressor T cells which depress the delayed footpad reaction to sheep erythrocytes (SRBC) was studied in mice in which MPS was blocked or stimulated. Colloidal carbon and diethylstilbestrol were used for blockade and stimulation respectively. Adoptive transfer of suppressor T cells was achieved by spleen cells of mice immunized intraperitoneally with varying doses of SRBC. In nontreated mice, 1 x 10(9) SRBC were required to induce suppressor T cells, while 6 x 10(8) could not induce the suppressor T cells. In MPS-blocked mice, however, even 6 x 10(8) SRBC could induce the suppressor T cells. On the other hand, 3 x 10(10) SRBC were required for the induction of suppressor T cells in MPS-stimulated mice. These results suggest that the activity of macrophages as scavenger cells modulates the subsequent induction of suppressor T cells after immunization with high doses of SRBC.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. IA antigens and antigen-presenting function of thymic macrophages. J Immunol. 1980 Mar;124(3):1433–1440. [PubMed] [Google Scholar]
- Chaouat G., Howard J. G. Influence of reticuloendothelial blockade on the induction of tolerance and immunity by polysaccharides. Immunology. 1976 Feb;30(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Das S., Leskowitz S. The cellular basis for tolerance or immunity to bovine-gamma-globulin in mice. J Immunol. 1974 Jan;112(1):107–114. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Wilkinson A., Wong M. Antigen-specific murine T-cell proliferation: role of macrophage surface Ia and factors. Cell Immunol. 1979 Nov;48(1):79–90. doi: 10.1016/0008-8749(79)90101-1. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. I. T suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1977 Oct;7(10):714–718. doi: 10.1002/eji.1830071013. [DOI] [PubMed] [Google Scholar]
- Lukić M. L., Cowing C., Leskowitz S. Strain differences in ease of tolerance induction to bovine gamma-globulin: dependence on macrophage function. J Immunol. 1975 Jan;114(1 Pt 2):503–506. [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS E. H., MAKINODAN T. THE SUPPRESSIVE ROLE OF MOUSE PERITONEAL PHAGOCYTES IN AGGLUTININ RESPONSE. J Immunol. 1965 May;94:765–777. [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A., Wood D. D., Benacerraf B. Immune responses in vitro. X. Functions of macrophages. J Immunol. 1974 Mar;112(3):1181–1189. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Paul W. E. Interaction between antigen-presenting cells and primed T lymphocytes: an assessment of Ir gene expression in the antigen-presenting cell. Immunol Rev. 1978;40:153–180. doi: 10.1111/j.1600-065x.1978.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Kishimoto S. Distinction between suppressors of the delayed-type hypersensitivity and the humoral response to sheep erythrocytes. Immunology. 1978 Nov;35(5):721–731. [PMC free article] [PubMed] [Google Scholar]
- Yamashita U., Shevach E. M. The expression of Ia antigens on immunocompetent cells in the guinea pig. II. Ia antigens on macrophages. J Immunol. 1977 Nov;119(5):1584–1588. [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Effect of stimulation and blockade of mononuclear phagocyte system on the delayed footpad reaction to SRBC in mice. Immunology. 1979 Nov;38(3):577–583. [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Relationship between non-specific activity of macrophages and immune responses to Listeria monocytogenes. Immunology. 1980 Jul;40(3):295–301. [PMC free article] [PubMed] [Google Scholar]


