Abstract
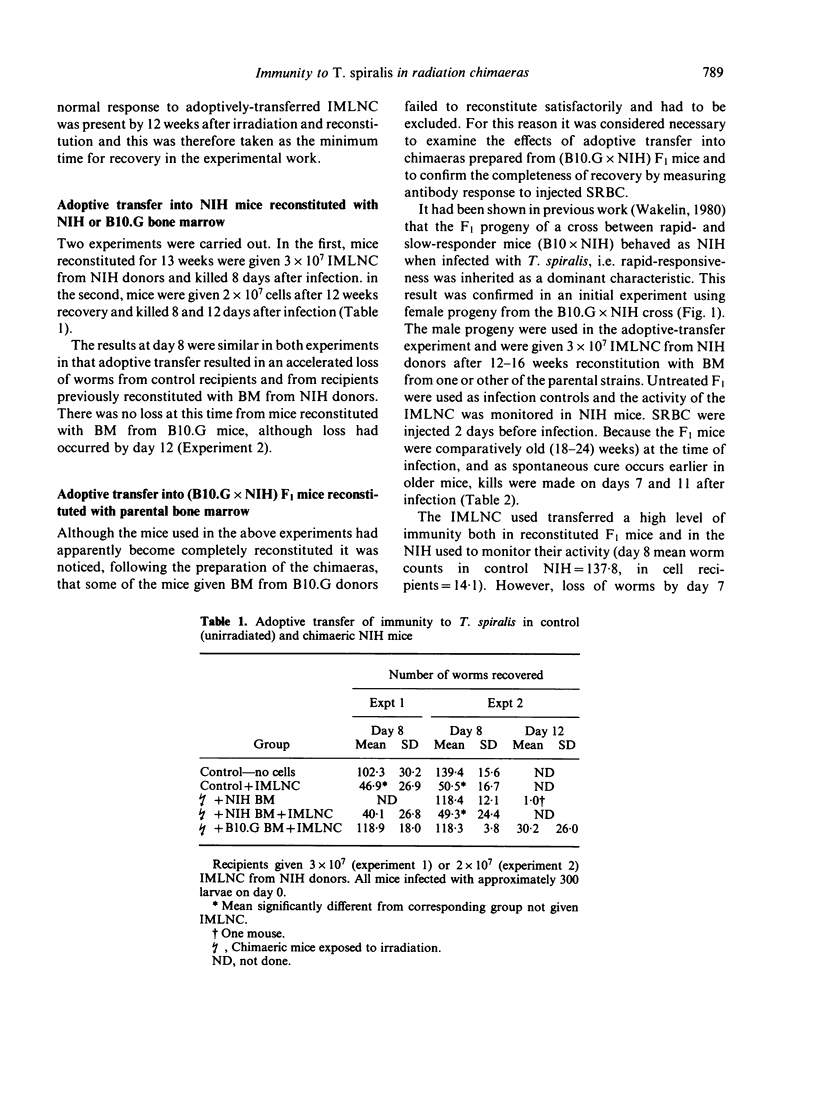
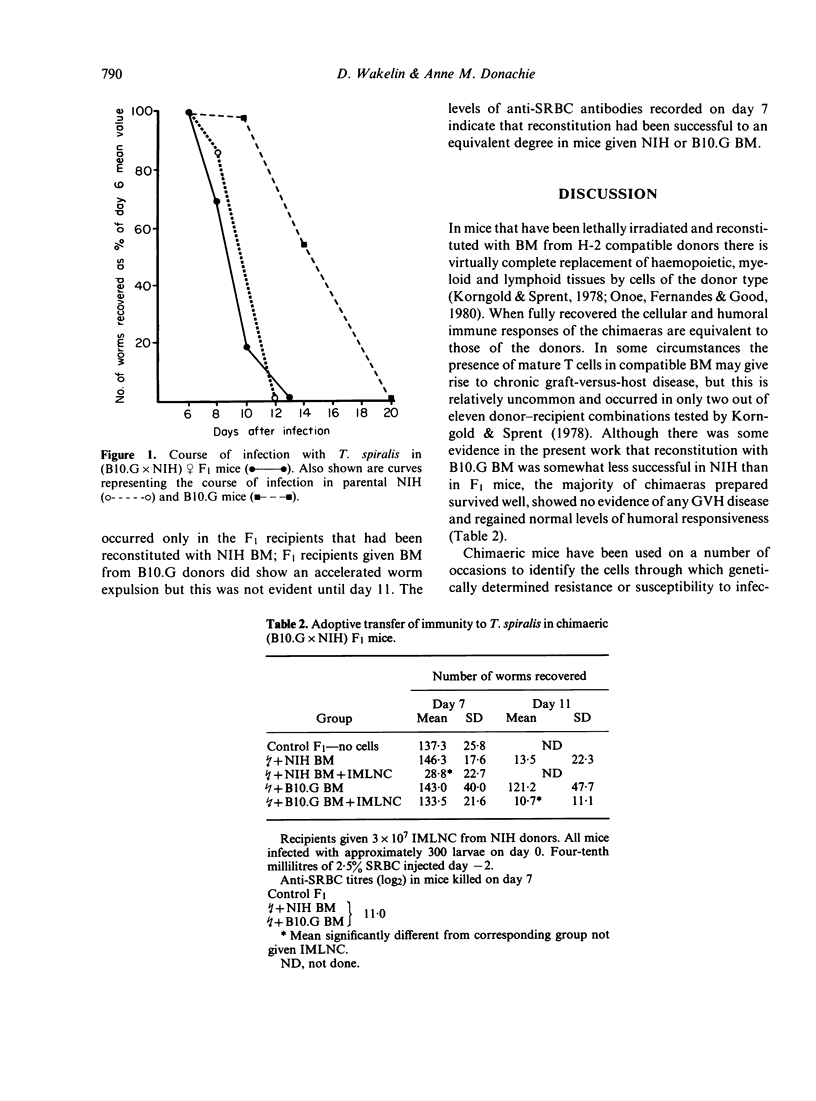
Radiation chimaeras, prepared from NIH (rapid-responder) mice or from the F1 progeny of a cross between H-2 compatible B10.G (slow-responder) and NIH mice, were tested for their ability to respond to infection with the intestinal nematode parasite Trichinella spiralis. Mice reconstituted with bone marrow (BM) from NIH donors showed the rapid response characteristic of this strain, i.e. expelled worms from the intestine before day 12 of infection; those given BM from B10.G mice showed a show expulsion pattern, losing worms after day 12. There was no evidence that the environment of the recipient exerted any influence on the ability of the BM cells to express the response characteristic of the donor. When chimaeras were given immune mesenteric lymph node cells (IMLNC) from infected NIH donors there was successful adoptive transfer of immunity, resulting in an accelerated loss of worms. As before, the time course of the accelerated response was determined by the genotype of the BM used. These results confirm that genetic control of the process of worm expulsion is expressed at the level of a bone marrow-derived cell population and is independent of lymphocyte responsiveness. They further show that the factors involved are an inherent property of the cells concerned. The possibility that these cells are myeloid in nature is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. The natural resistance of radiation chimeras to S. typhimurium C5. Immunology. 1979 Jun;37(2):329–332. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined susceptibility to Leishmania tropica infection is expressed by haematopoietic donor cells in mouse radiation chimaeras. Nature. 1980 Nov 13;288(5787):161–162. doi: 10.1038/288161a0. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Sadarangani C., Skamene E. Genetically determined differences in antibacterial activity of macrophages are expressed in the environment in which the macrophage precursors mature. Cell Immunol. 1980 Aug 1;53(2):341–349. doi: 10.1016/0008-8749(80)90334-2. [DOI] [PubMed] [Google Scholar]
- Korngold R., Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J Exp Med. 1978 Dec 1;148(6):1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F. Responses to infection with metazoan and protozoan parasites in mice. Adv Immunol. 1979;28:451–511. doi: 10.1016/s0065-2776(08)60803-2. [DOI] [PubMed] [Google Scholar]
- Onoé K., Fernandes G., Good R. A. Humoral and cell-mediated immune responses in fully allogeneic bone marrow chimera in mice. J Exp Med. 1980 Jan 1;151(1):115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to parasites: adoptive transfer of immunity between inbred strains of mice characterized by rapid and slow immune expulsion of Trichinella spiralis. Parasite Immunol. 1980 Winter;2(4):249–260. doi: 10.1111/j.1365-3024.1980.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Wakelin D. Genetic control of susceptibility and resistance to parasitic infection. Adv Parasitol. 1978;16:219–308. doi: 10.1016/s0065-308x(08)60575-8. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Lloyd M. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology. 1976 Apr;72(2):173–182. doi: 10.1017/s0031182000048472. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. Immunity to Trichinella spiralis in irradiated mice. Int J Parasitol. 1980 Feb;10(1):37–41. doi: 10.1016/0020-7519(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. T and B cells in the transfer of immunity against Trichinella spiralis in mice. Immunology. 1979 May;37(1):103–109. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. Transfer of immunity to Trichinella spiralis in the mouse with mesenteric lymph node cells: time of appearance of effective cells in donors and expression of immunity in recipients. Parasitology. 1977 Jun;74(3):215–224. doi: 10.1017/s0031182000047843. [DOI] [PubMed] [Google Scholar]
- Walls R. S., Carter R. L., Leuchars E., Davies A. J. The immunopathology of trichiniasis in T-cell deficient mice. Clin Exp Immunol. 1973 Feb;13(2):231–242. [PMC free article] [PubMed] [Google Scholar]


