Abstract
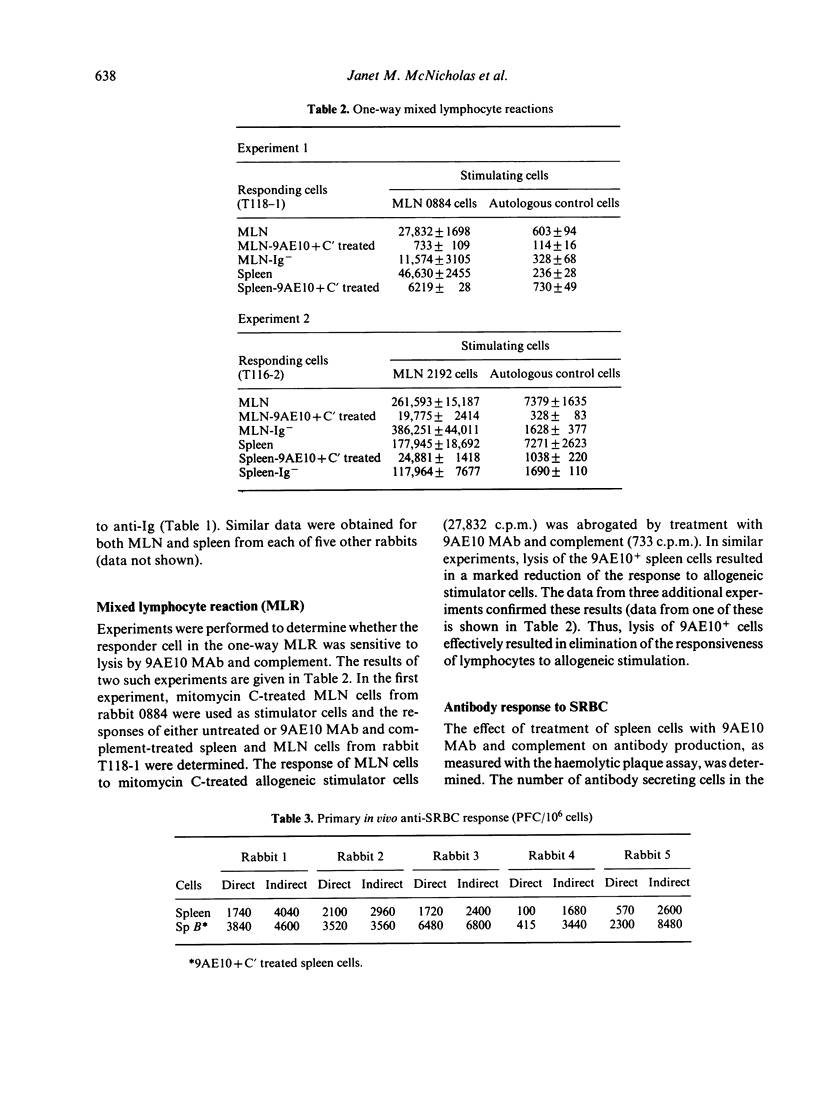
Rabbit spleen and mesenteric lymph node cells were treated with a monoclonal anti-rabbit T-lymphocyte antibody (MAb) and complement and the effect of the treatment on various lymphocyte functions was determined. Lysis of spleen and mesenteric lymph node cells reactive with this MAb, 9AE10, essentially eliminated their proliferative responsiveness to allogeneic lymphocytes in the mixed lymphocyte reaction and to the T-cell mitogens, concanavalin A and phytohaemagglutinin; responsiveness to the B-cell mitogen, anti-immunoglobulin (Ig) was not decreased by lysis of 9AE10+ cells. In addition, the 9AE10+ cells were found to be necessary for the secondary in vitro antibody response to the T-dependent antigen sheep red blood cells (SRBC), as removal of 9AE10+ cells blocked the generation of plaque forming cells (PFC) in culture. The PFC's themselves were not sensitive to lysis by 9AE10 MAb and complement Thus, the 9AE10 MAb appears to recognize cells which have functions characteristic of T lymphocytes and this monoclonal antibody will be useful in further studies of the rabbit cellular immune system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Segall M. Genetics of the mixed leukocyte culture response: a reexamination. Transplant Proc. 1972 Jun;4(2):205–208. [PubMed] [Google Scholar]
- Fanger M. W., Pelley R. P., Reese A. L. In vitro demonstration of two antigenically-distinct rabbit lymphocyte populations. J Immunol. 1972 Aug;109(2):294–303. [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. In vitro responses of rat lymphocytes following adult thymectomy. I. Rapid decrease and recovery of responses to mitogens and hemiallogeneic cells. J Immunol. 1972 Nov;109(5):1046–1051. [PubMed] [Google Scholar]
- Fradelizi D. P., Chou C. T., Cinader B., Dubiski S. A membrane antigen of rabbit thymus cells. Cell Immunol. 1973 Jun;7(3):484–501. doi: 10.1016/0008-8749(73)90212-8. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Ozer H., Jr, Waksman B. H. The response of rabbit lymphocytes to mitogens and alloantigens: evidence for T cell heterogeneity. J Immunol. 1974 Dec;113(6):1780–1792. [PubMed] [Google Scholar]
- Ozer H., Waksman B. H. Appendix and M antibody formation. V. Appendix and thymus cell synergism in the direct and indirect plaque-forming cell responses to sheep erythrocytes in the rabbit. J Immunol. 1972 Aug;109(2):410–412. [PubMed] [Google Scholar]
- Plate J. M., McKenzie I. F. "B"-cell stimulation of allogeneic T-cell proliferation in mixed lymphocyte cultures. Nat New Biol. 1973 Oct 24;245(147):247–249. doi: 10.1038/newbio245247a0. [DOI] [PubMed] [Google Scholar]
- Redelman D., Scott C. B., Sheppard H. W., Jr, Sell S. In vitro studies of the rabbit immune system: II. Functional characterization of rabbit T and B populations separated by adherence to nylon wool or lysis with anti-thymocyte serum and complement. Cell Immunol. 1976 Jun 1;24(1):11–23. doi: 10.1016/0008-8749(76)90127-1. [DOI] [PubMed] [Google Scholar]
- Sell S., Sheppard H. W., Jr Rabbit blood lymphocytes may be T cells with surface immunoglobulins. Science. 1973 Nov 9;182(4112):586–587. doi: 10.1126/science.182.4112.586. [DOI] [PubMed] [Google Scholar]
- Shek P. N., Chou C. T., Dubiski S., Cinader B. Mitogen stimulation of rabbit spleen cells before and after complement-mediated cell kill with an antiserum directed against the thymus antigen RTLA. Int Arch Allergy Appl Immunol. 1974;46(5):753–767. doi: 10.1159/000231175. [DOI] [PubMed] [Google Scholar]
- Shek P. N., Coons A. H. Effect of colchicine on the antibody response. I. Enhancement of antibody formation in mice. J Exp Med. 1978 Apr 1;147(4):1213–1227. doi: 10.1084/jem.147.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard H. W., Jr, Redelman D., Sell S. In vitro studies of the rabbit immune system. IV. Differential mitogen responses of isolated T and B cells. Cell Immunol. 1976 Jun 1;24(1):34–44. doi: 10.1016/0008-8749(76)90129-5. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Jr, Sell S., Poler S. M., Redelman D. D. Mixed lymphocyte reactions in the rabbit using peripheral blood cells: the effects of cell preparation and skin grafting. J Immunol Methods. 1977;16(2):185–196. doi: 10.1016/0022-1759(77)90052-7. [DOI] [PubMed] [Google Scholar]
- Stavitsky A. B., Cook R. G. In vitro anamnestic response of rabbit lymph node cells: evidence for cell collaboration in induction and regulation. J Immunol. 1974 Feb;112(2):583–593. [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Teodorescu M., Mayer E. P., Reiter H., Dray S. Rabbit lymphocyte subpopulations. I. Separation of Ig+ and Ig- cells and their interaction in cultures stimulated by mitogens. Cell Immunol. 1976 Mar 1;22(1):66–75. doi: 10.1016/0008-8749(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Tyan M. L., Ness D. B. Modification of the mixed leukocyte reaction with various antisera. Transplantation. 1972 Feb;13(2):198–201. doi: 10.1097/00007890-197202000-00027. [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Gurner B. W., Coombs R. R. Observations on rabbit thymocytes and peripheral T cells. II. Rosette formation with rabbit erythrocytes. Int Arch Allergy Appl Immunol. 1975;48(3):383–394. doi: 10.1159/000231323. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Teodorescu M., Dray S. Enumeration and isolation of rabbit T and B lymphocytes by using antibody-coated erythrocytes. J Immunol. 1976 May;116(5):1306–1312. [PubMed] [Google Scholar]
- Zimmerman D. H., Okumura K., Rabkin C., Kern M. Differentiation of lymphoid cells: the use of specific antisera to characterize the cells required for the induction of immunoglobulin M production in vitro. J Immunol. 1974 Dec;113(6):1891–1896. [PubMed] [Google Scholar]


