Abstract
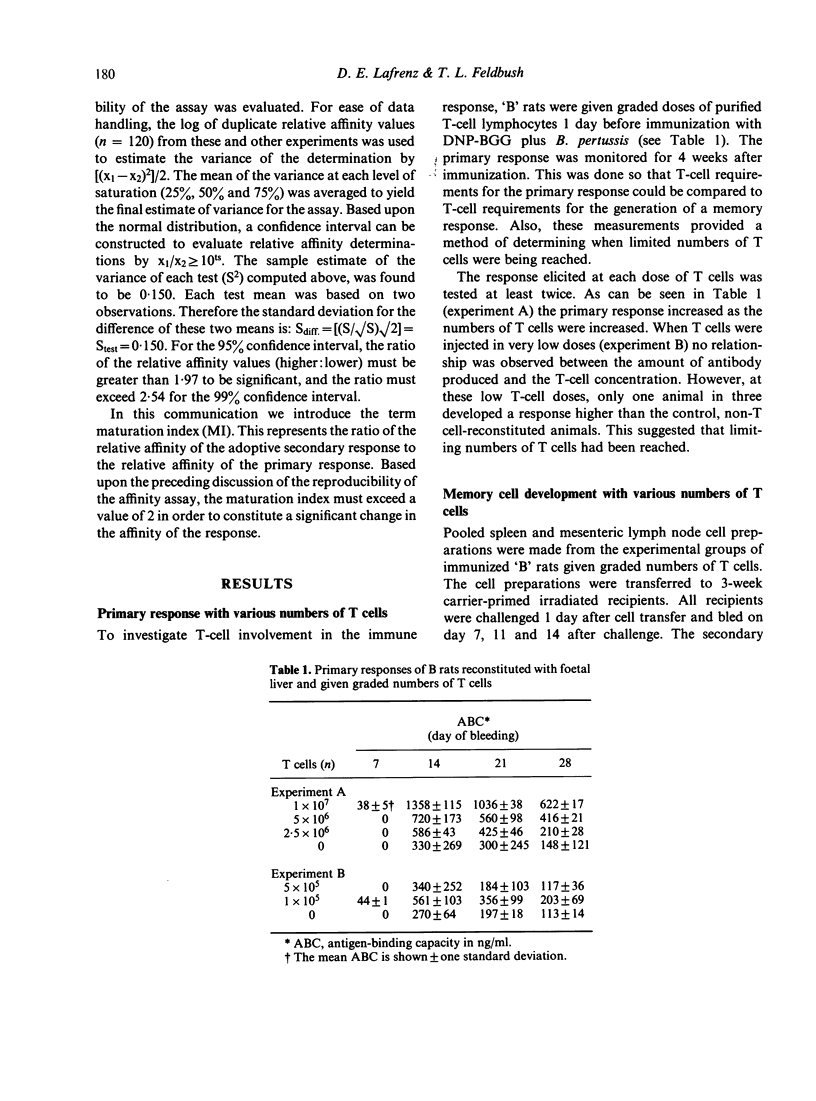
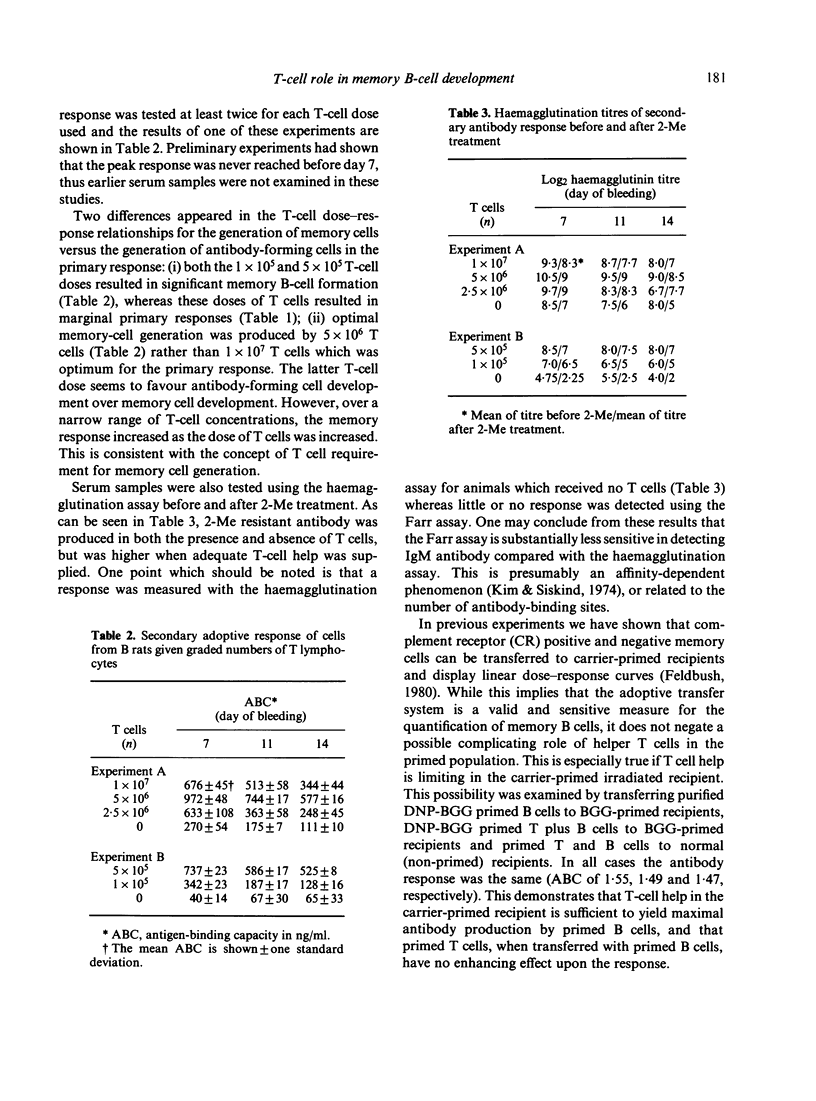
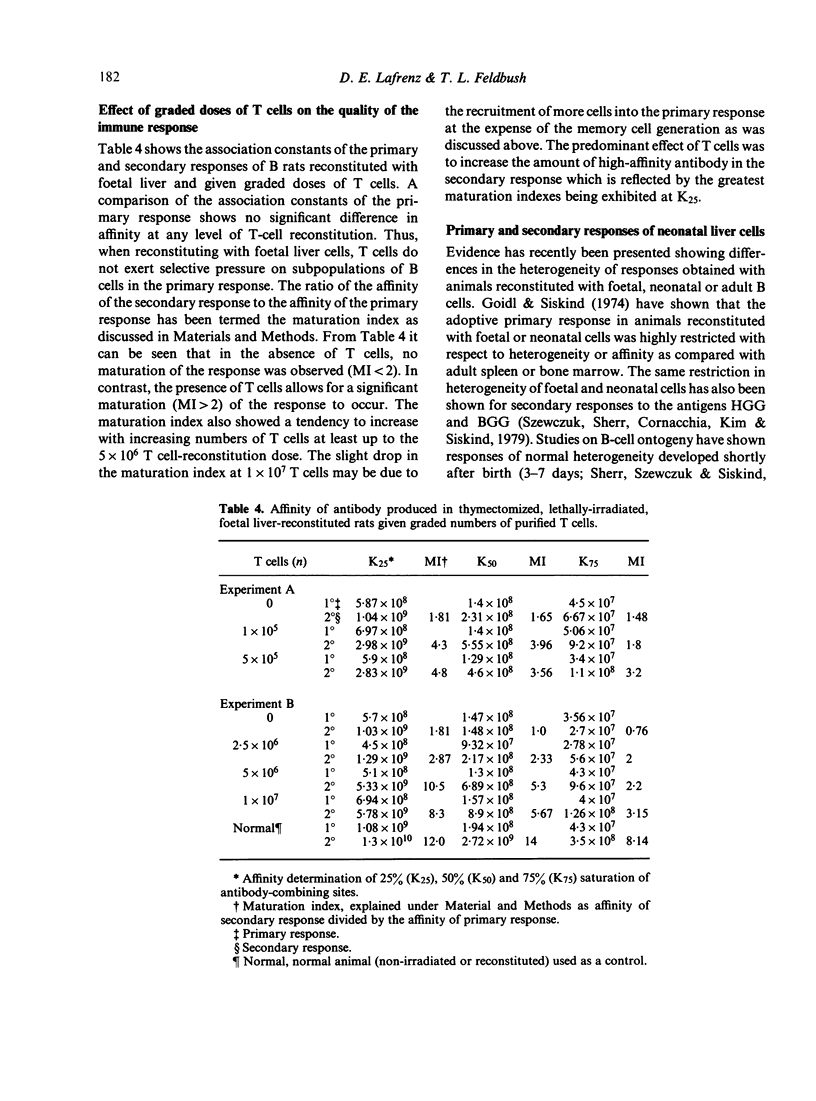
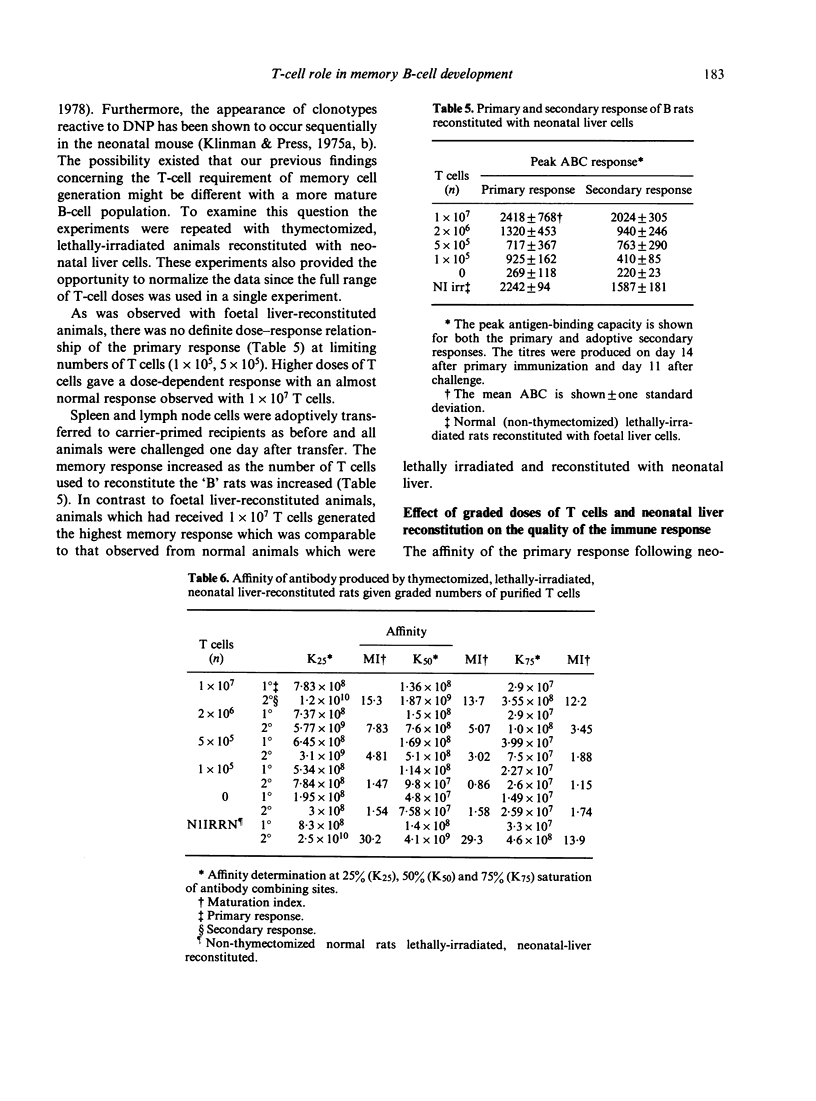
The purpose of this investigation was to address the current controversy regarding the T-cell requirement for the generation of B-memory cells. We have circumvented the possible objection to previous experiments regarding residual T cells in T-deprived animals by examining memory cell generation in relation to the numbers of T cells participating in the immune response. Thymectomized and lethally-irradiated rats were reconstituted with foetal liver or a more mature stem cell source, neonatal liver. These animals were given graded doses of purified T cells one day before immunization with alum-precipitated DNP-BGG + Bordetella pertussis. Four weeks after priming, cell suspensions from experimental groups were adoptively transferred to carrier primed recipients and challenged with DNP-BGG in saline to assess memory cell development. The data demonstrate that in the absence of T cells only minimal memory development occurred. However, when T cells were present, the level of memory cell development increased with increasing numbers of T cells. By examining the relative affinity of the antibody produced in the primary and secondary responses, the increase in memory cell development in relation to increased numbers of T cells was shown to be due to the selective generation of high affinity memory B cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braley-Mullen H. Secondary IgG responses to type 3 pneumococcal polysaccharide. III. T cell requirement for development of B memory cells. Eur J Immunol. 1977 Nov;7(11):775–781. doi: 10.1002/eji.1830071106. [DOI] [PubMed] [Google Scholar]
- DeKruyff R., Siskind G. W. Studies on the control of antibody synthesis. XIV. Role of T cells in regulating antibody affinity. Cell Immunol. 1979 Sep 15;47(1):134–142. doi: 10.1016/0008-8749(79)90321-6. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. The influence of T cells on the initiation and expression of immunological memory. Immunology. 1979 Oct;38(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W. The role of T cells and adjuvant in the immune response of mice to foreign erythrocytes. Eur J Immunol. 1972 Feb;2(1):50–57. doi: 10.1002/eji.1830020111. [DOI] [PubMed] [Google Scholar]
- Feldbush T. L. Antigen modulation of the immune response. The decline of immunological memory in the absence of continuing antigenic stimulation. Cell Immunol. 1973 Sep;8(3):435–444. doi: 10.1016/0008-8749(73)90134-2. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Goidl E. A., Siskind G. W. Ontogeny of B-lymphocyte function. I. Restricted heterogeneity of the antibody response of B lymphocytes from neonatal and fetal mice. J Exp Med. 1974 Nov 1;140(5):1285–1302. doi: 10.1084/jem.140.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. T., Kalver S., Siskind G. W. A comparison of the Farr technique with equilibrium dialysis for measurement of antibody concentration and affinity. J Immunol Methods. 1975 Feb;6(4):347–354. doi: 10.1016/0022-1759(75)90005-8. [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Siskind G. W. Studies on the control of antibody synthesis. VI. Effect of antigen dose and time after immunization on antibody affinity and heterogeneity in the mouse. Clin Exp Immunol. 1974 Jun;17(2):329–338. [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Press J. L. Expression of specific clones during B cell development. Fed Proc. 1975 Jan;34(1):47–50. [PubMed] [Google Scholar]
- Klinman N. R., Press J. L. The characterization fo the B-cell repertoire specific for the 2,4-dinitrophenyl and 2,4,6-trinitrophenyl determinants in neonatal BALB/c mice. J Exp Med. 1975 May 1;141(5):1133–1146. doi: 10.1084/jem.141.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The effect of Bordetella pertussis on the antibody response in mice to type III pneumococcal polysaccharide. J Immunol. 1976 Apr;116(4):989–993. [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Cell-to-cell interaction in the immune response. VI. Contribution of thymus-derived cells and antibody-forming cell precursors to immunological memory. J Exp Med. 1971 Jul 1;134(1):66–82. doi: 10.1084/jem.134.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Grumet F. C., McDevitt H. O. Genetic control of the immune response. The effect of thymectomy on the primary and secondary antibody response of mice to poly-L(tyr, glu)-poly-D, L-ala--poly-L-lys. J Exp Med. 1972 Jan;135(1):126–135. doi: 10.1084/jem.135.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Metzler C. M., Tsu T. T., Herzenberg L. A., Herzenberg L. A. Two stages of B-cell memory development with different T-cell requirements. J Exp Med. 1976 Aug 1;144(2):345–357. doi: 10.1084/jem.144.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano T. J., Thorbecke G. J. Thymus influence on the conversion of 19S to 7S antibody formation in the response to TNP-Brucella. J Immunol. 1975 Aug;115(2):332–334. [PubMed] [Google Scholar]
- Sanfilippo F., Scott D. W. The effects of carrier-specific helper T-cell tolerance on antibody avidity in the anti-hapten response. Cell Immunol. 1976 Jan;21(1):112–120. doi: 10.1016/0008-8749(76)90332-4. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A. Antigen-initiated B lymphocyte differentiation. Kinetics and T lymphocyte dependence of the primary and secondary in vitro immune responses to the hapten 4-hydroxy-3-iodo-5-nitrophenylacetic acid presented on the carrier polymerised bacterial flagellin. Aust J Exp Biol Med Sci. 1974 Jun;52(Pt 3):471–489. [PubMed] [Google Scholar]
- Schrader J. W. The role of T cells in IgG production; thymus-dependent antigens induce B cell memory in the absence of T cells. J Immunol. 1975 Jun;114(6):1665–1669. [PubMed] [Google Scholar]
- Schrater A. F., Goidl E. A., Thorbecke G. J., Siskind G. W. Production of auto-anti-idiotypic antibody during the normal immune response to TNP-ficoll. I. Occurrence in AKR/J and BALB/c mice of hapten-augmentable, anti-TNP plaque-forming cells and their accelerated appearance in recipients of immune spleen cells. J Exp Med. 1979 Jul 1;150(1):138–153. doi: 10.1084/jem.150.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr D. H., Szewczuk M. R., Siskind G. W. Ontogeny of B-lymphocyte function. V. Thymus cell involvement in the functional maturation of B-lymphocytes from fetal mice transferred into adult irradiated hosts. J Exp Med. 1978 Jan 1;147(1):196–206. doi: 10.1084/jem.147.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E., Cantor H. Regulation of the immune response by subclasses of T lymphocytes. II. The effect of adult thymectomy upon humoral and cellular responses in mice. Eur J Immunol. 1975 May;5(5):337–343. doi: 10.1002/eji.1830050509. [DOI] [PubMed] [Google Scholar]
- Szewczuk M. R., Sherr D. H., Cornacchia A., Kim Y. T., Siskind G. W. Ontogeny of B lymphocyte function. XI. The secondary response by neonatal and adult B cell populations to different T-dependent antigens. J Immunol. 1979 Apr;122(4):1294–1301. [PubMed] [Google Scholar]
- Torrigiani G. Quantitative estimation of antibodies in the immunoglobulin classes of the mouse. I. Effect of adjuvants on the antibody response to human serum albumin and keyhole limpet haemocyanin. Clin Exp Immunol. 1972 May;11(1):125–135. [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Kim Y. T., Quagliata F., Siskind G. W. Studies on the control of antibody synthesis. 3. Changes in heterogeneity of antibody affinity during the course of the immune response. Immunology. 1973 Mar;24(3):477–492. [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Siskind G. W. Distribution of antibody affinities: technique of measurement. Immunochemistry. 1972 Oct;9(10):987–1011. doi: 10.1016/0019-2791(72)90110-3. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Waksman B. H. Use of thymectomized, irradiated rats to study immunogenicity of bovine gamma globulin. J Immunol. 1969 Apr;102(4):925–931. [PubMed] [Google Scholar]


