Abstract
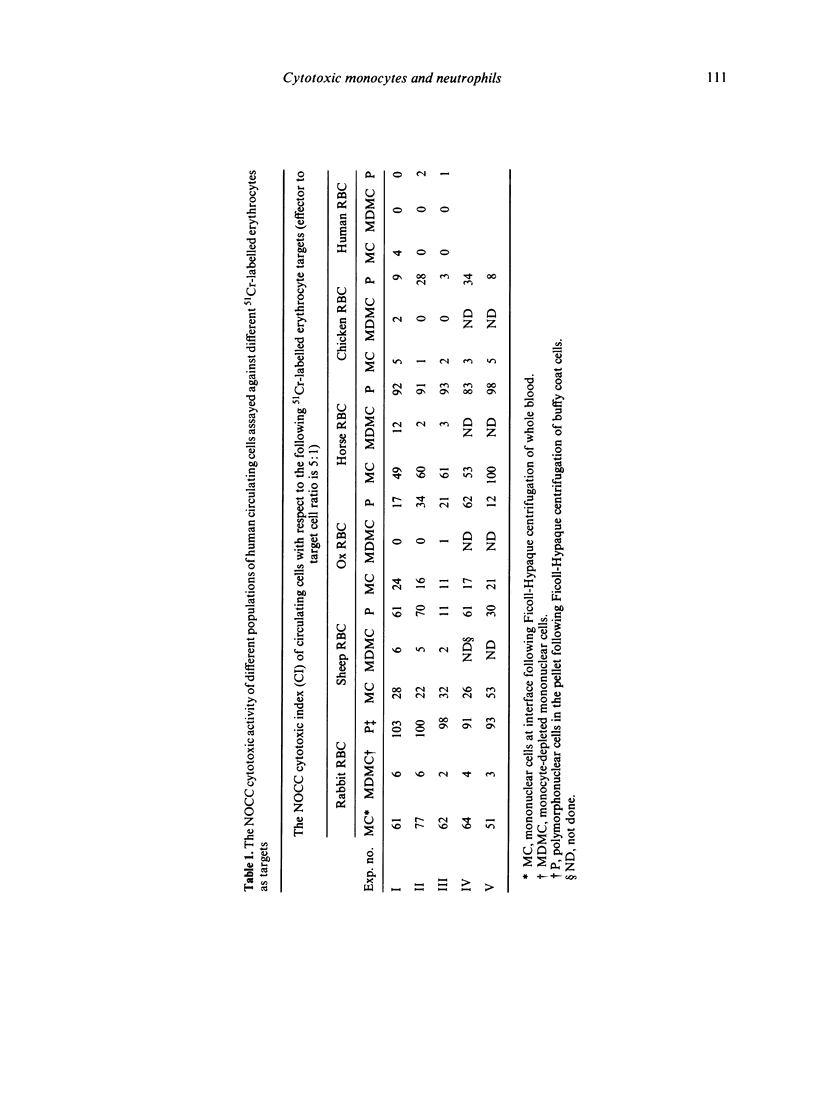
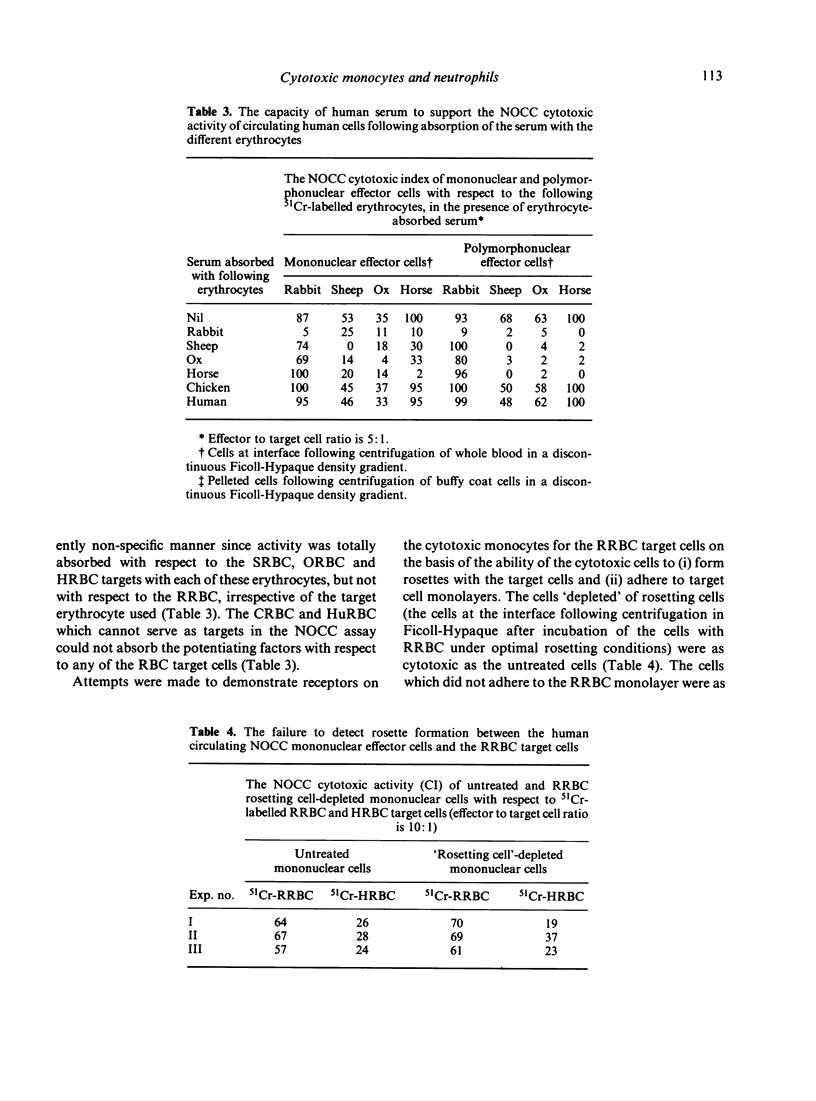
The naturally-occurring antibody-independent cellular cytotoxic activity (NOCC) of normal circulating human monocytes and neutrophils was investigated employing a number of erythrocytes and the K-562 cell line as target cells simultaneously. The identity of the effector cell(s) was shown to be dependent upon or be a function of the type of target cell selected for the assay system. A number of erythrocyte targets (rabbit, horse, sheep and ox erythrocytes) were lysed to varying degrees by neutrophils and monocytes and not by lymphocytes. Irrespective of the red blood cell (RBC) target, the effector monocyte invariably possessed receptors for both C'3 and the Fc of IgG. In contrast, the cytotoxic cells using the K-562 target cell were lymphocytes. Monocytes and neutrophils were inactive. The cytotoxic-enhancing activity in normal human serum exhibits specific and non-specific properties which suggests that more than one factor is involved. With respect to the monocyte cytotoxic cells, only the rabbit erythrocytes could totally absorb the serum factor in a specific fashion. Absorption of the serum with horse, sheep or ox erythrocytes resulted in a significant loss of potentiating activity with respect to all of the erythrocyte targets but a more marked loss of activity using the absorbing erythrocytes as targets. With respect to the polymorphonuclear leucocyte effector cells, only the rabbit RBC were capable of specifically absorbing out the cytotoxic-enhancing factor present in the normal human serum. Absorption of the serum with sheep, horse or ox RBC resulted in total cross-absorption of the enhancing factor. Chicken and human RBC, which do not serve as targets for the NOCC assay, could not absorb out the cytotoxic-enhancing factor with respect to any of the target erythrocytes. The composition of the soluble serum factor(s) is under current investigation but it is not an immunoglobulin since pure serum albumin can substitute for normal serum in the NOCC assay. The mechanism of erythrocyte lysis by the cytotoxic monocyte was investigated. Mononuclear cells were incubated with target cell monolayers and with target cells under optimal rosetting conditions. No interaction between the effector and target cells could be detected. The monocytes did not adhere to the target cell monolayer nor did they form rosettes with the target cells. Thus, the results fail to corroborate or support the assumption that the cytotoxic activity of the monocyte is dependent upon conventionally-detectable receptors. Erythrophagocytosis was not observed to any significant degree under the assay conditions used. Therefore, the nature of the interaction between the cytotoxic monocyte and the erythroid target cell which results in lysis of the target cell remains to be elucidated.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakacs T., Gergely P., Klein E. Characterization of cytotoxic human lymphocyte subpopulations: the role of Fc-receptor-carrying cells. Cell Immunol. 1977 Aug;32(2):317–328. doi: 10.1016/0008-8749(77)90208-8. [DOI] [PubMed] [Google Scholar]
- Behelak Y., Richter M. Cells involved in cell-mediated and transplantation immunity in the rabbit. 8. The killer cell activity of the lymphocytes in the gastrointestine-associated lymphoid organs of the normal unsensitized adult rabbit. Transplantation. 1974 Sep;18(3):229–238. doi: 10.1097/00007890-197409000-00005. [DOI] [PubMed] [Google Scholar]
- Behelak Y., Richter M. Cells involved in cell-mediated and transplantation immunity. 3. The organ source(s) of the cells in the normal rabbit which mediate a reaction of cellular immunity in vitro. Cell Immunol. 1972 Apr;3(4):542–558. doi: 10.1016/0008-8749(72)90118-9. [DOI] [PubMed] [Google Scholar]
- Behelak Y., Shibata H., Richter M. Cells involved in cell-mediated and transplantation immunity in the rabbit. V. The loss of effector cells following extirpation of the spleen or the appendix, sacculus rotundus, and Peyer's patches. Cell Immunol. 1973 Apr;7(1):108–117. doi: 10.1016/0008-8749(73)90186-x. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Ojo E. A., Orn A., Wigzell H., Murgita R. A. Severe suppression of the B-cell system has no impact on the maturation of natural killer cells in mice. Scand J Immunol. 1979;9(2):167–173. doi: 10.1111/j.1365-3083.1979.tb02719.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Edwards J., Adams E., Milton G. W., Nelson D. S. Specificity of cell-mediated cytotoxicity against human melanoma lines: evidence for "non-specific" killing by activated T-cells. Int J Cancer. 1975 Jul 15;16(1):173–183. doi: 10.1002/ijc.2910160119. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Kight N., Temple A., Allison A. C. Spontaneous and induced cytotoxic properties of human adherent mononuclear cells: killing of non-sensitized and antibody-coated non-erythroid cells. Immunology. 1979 Feb;36(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Kall M. A., Koren H. S. Heterogeneity of human natural killer cell populations. Cell Immunol. 1978 Sep 15;40(1):58–68. doi: 10.1016/0008-8749(78)90315-5. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Kärre K., Jondal M., Tracey D., Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976 Apr 1;143(4):772–780. doi: 10.1084/jem.143.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi M., Takasugi M. The nonselective cytotoxic cell (N cell). J Natl Cancer Inst. 1976 Mar;56(3):575–582. doi: 10.1093/jnci/56.3.575. [DOI] [PubMed] [Google Scholar]
- Melsom H., Seljelid R. The cytotoxic effect of mouse macrophages on syngeneic and allogeneic erythrocytes. J Exp Med. 1973 Mar 1;137(3):807–820. doi: 10.1084/jem.137.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Spontaneous cytotoxicity of human peripheral mononuclear cells toward red blood cell targets in vitro. I. characterization of the killer cell. J Immunol. 1977 Nov;119(5):1680–1685. [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Synergistic cytotoxicity. I. Characterization of a heat labile plasma fraction that induces nonspecific cytotoxicity by human mononuclear cells. J Immunol. 1979 Mar;122(3):1146–1151. [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Synergistic cytotoxicity. II. In vitro arming of monocytes and T cells by a heat labile fraction of human plasma. J Immunol. 1979 Mar;122(3):1152–1155. [PubMed] [Google Scholar]
- Nelson D. L., Bundy B. M., Strober W. Spontaneous cell-mediated cytotoxicity by human peripheral blood lymphocytes in vitro. J Immunol. 1977 Oct;119(4):1401–1405. [PubMed] [Google Scholar]
- Ortaldo J. R., Bonnard G. D., Kind P. D., Herberman R. B. Cytotoxicity by cultured human lymphocytes: characteristics of effector cells and specificity of cytotoxicity. J Immunol. 1979 Apr;122(4):1489–1494. [PubMed] [Google Scholar]
- Pape G. R., Moretta L., Troye M., Perlmann P. Natural cytotoxicity of human Fc gamma-receptor-positive T lymphocytes after surface modulation with immune complexes. Scand J Immunol. 1979 Mar;9(3):291–296. doi: 10.1111/j.1365-3083.1979.tb02734.x. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Comparison of the effector cells in human spontaneous cellular cytotoxicity and antibody-dependent cellular cytotoxicity: differential sensitivity of effector cells to in vivo and in vitro corticosteroids. Scand J Immunol. 1978;8(2):99–107. doi: 10.1111/j.1365-3083.1978.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Potter M. R., Moore M. Natural cytotoxic reactivity of human lymphocyte subpopulations. Immunology. 1979 May;37(1):187–194. [PMC free article] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Jondal M. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. II. Is the complement receptor necessarily present on the killer cells? Int J Cancer. 1977 Sep 15;20(3):353–358. doi: 10.1002/ijc.2910200306. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Gupta S., Good R. A., Baines M. G. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. VII. The effect of immunodeficiency disease. Cell Immunol. 1979 Mar 1;43(1):160–175. doi: 10.1016/0008-8749(79)90159-x. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975 Aug;21(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R. Gross-virus-induced lymphoma in the rat. V. Natural cytotoxic cells are non-T cells. Int J Cancer. 1977 Feb 15;19(2):225–235. doi: 10.1002/ijc.2910190212. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Probert M., Thomas L. H. Cytotoxicity of alveolar macrophages for virus-infected cells. Nature. 1975 Jun 26;255(5511):710–712. doi: 10.1038/255710a0. [DOI] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- de Vries J. E., Cornain S., Rumke P. Cytotoxity of non-T versus T-lymphocytes from melanoma patients and healthy donors on short- and long-term cultured melanoma cells,. Int J Cancer. 1974 Oct 15;14(4):427–434. doi: 10.1002/ijc.2910140402. [DOI] [PubMed] [Google Scholar]


