Abstract
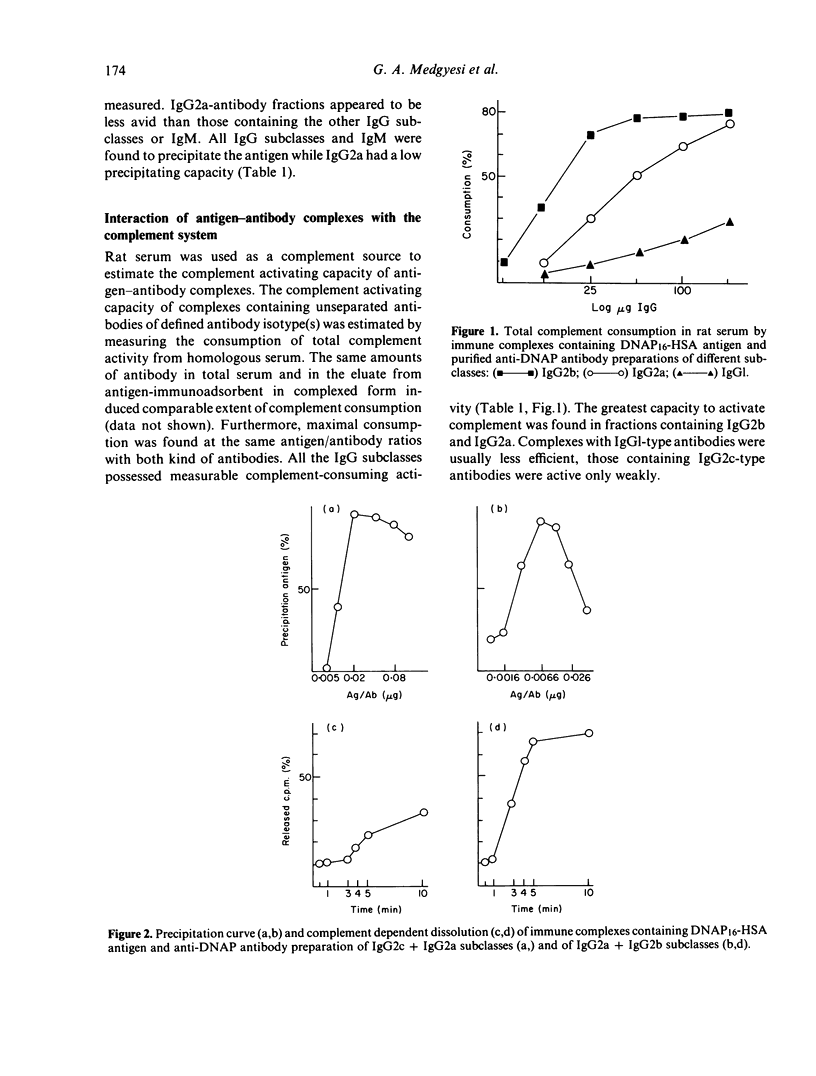
Antibodies against the dinitroaminophenyl (DNAP) haptenic group were raised in outbred CFY rats using HSA or LPS as carrier. Antibodies isolated by immunoadsorbent techniques were resolved into fractions representing distinct isotypes, and the resulting fractions were tested for avidity. Subclass IgG2a was found to contain antibodies of an avidity index lower than those of other IgG subclasses or IgM. IgG2a was the only isotype detected when DNAP-LPS was used for immunization. Complexes containing defined isotypes were compared for their capacity to activate homologous complement. IgGl type antibody-containing complexes displayed a low complement activating capacity compared with those containing IgG2b, IgG2c or IgG2a. The latter subclass when complexed with antigen can thus induce complement-dependent processes in spite of a low avidity. Insoluble complexes of IgG antibodies were rapidly solubilized in rat serum (CRA phenomenon), except those containing predominantly IgG2c.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celada F., Schmidt D., Strom R. Determination of avidity of anti-albumin antibodies in the mouse. Influence of the number of cells transferred on the quality of the secondary adoptive response. Immunology. 1969 Aug;17(2):189–198. [PMC free article] [PubMed] [Google Scholar]
- Chalon M. P., Milne R. W., Vaerman J. P. Interactions between mouse immunoglobulins and staphylococcal protein A. Scand J Immunol. 1979;9(4):359–364. doi: 10.1111/j.1365-3083.1979.tb03174.x. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Hyslop N. E., Jr, Dourmashkin R. R., Green N. M., Porter R. R. The fixation of complement and the activated first component (C1) of complement by complexes formed between antibody and divalent hapten. J Exp Med. 1970 Apr 1;131(4):783–802. doi: 10.1084/jem.131.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A., van Es L. A., Daha M. R. Effects of C-1 on the size of soluble immune aggregates and on their processing by macrophages. J Immunol. 1979 Aug;123(2):640–645. [PubMed] [Google Scholar]
- Klaus G. G. Generation of memory cells. III. Antibody class requirements for the generation of B-memory cells by antigen--antibody complexes. Immunology. 1979 Jun;37(2):345–351. [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Medgyesi G. A., Füst G., Gergely J., Bazin H. Classes and subclasses of rat immunoglobulins: interaction with the complement system and with staphylococcal protein A. Immunochemistry. 1978 Feb;15(2):125–129. doi: 10.1016/0161-5890(78)90052-4. [DOI] [PubMed] [Google Scholar]
- Medgyesi G. A., Füst G., Gergely J., Jaton J. C. Activation of the classical complement pathway by homogeneous anti-SIII antibody bound to bivalent or trivalent oligosaccharide antigens. Mol Immunol. 1979 Nov;16(11):949–956. doi: 10.1016/0161-5890(79)90096-8. [DOI] [PubMed] [Google Scholar]
- Rajnavölgyi E., Füst G., Kulics J., Ember J., Medgyesi G. A., Gergely J. The effect of immune complex composition on complement activation and complement dependent complex release. Immunochemistry. 1978 Dec;15(12):887–894. doi: 10.1016/0161-5890(78)90123-2. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Czop J., Ferreira A., Nussenzweig V. Mechanism of solubilization of immune aggregates by complement. Implications for immunopathology. Transplant Rev. 1976;32:121–139. doi: 10.1111/j.1600-065x.1976.tb00231.x. [DOI] [PubMed] [Google Scholar]


