Abstract
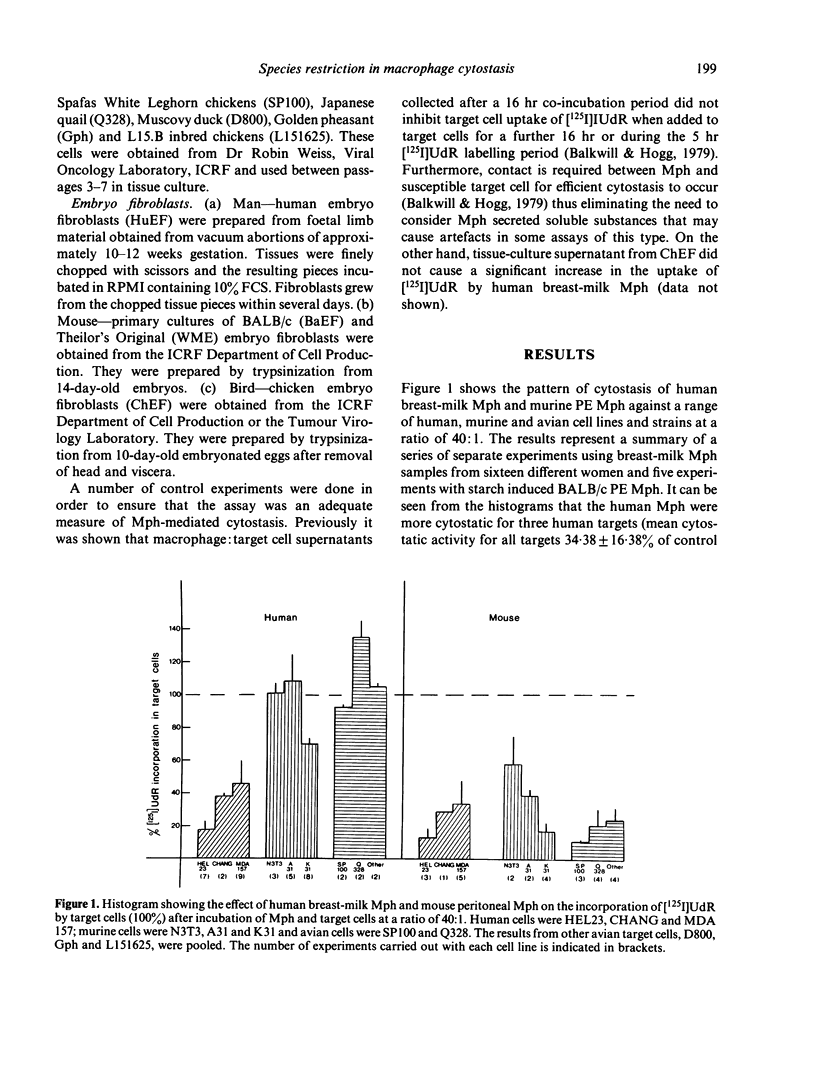
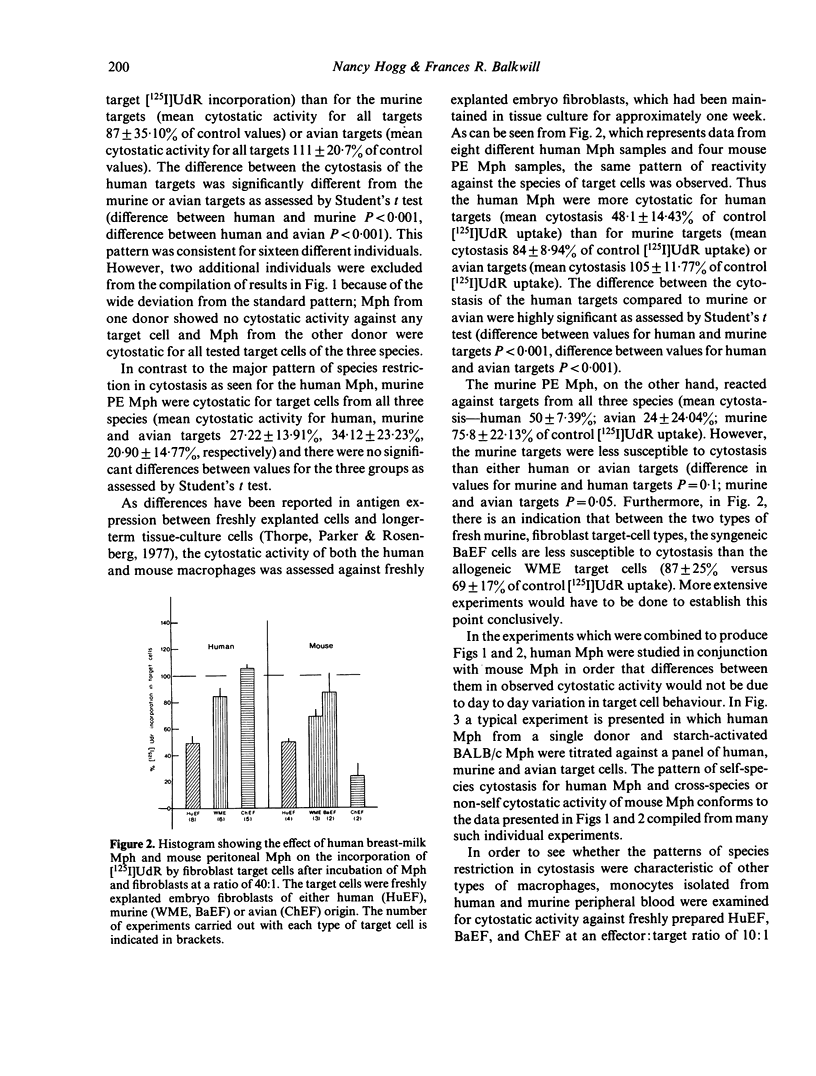
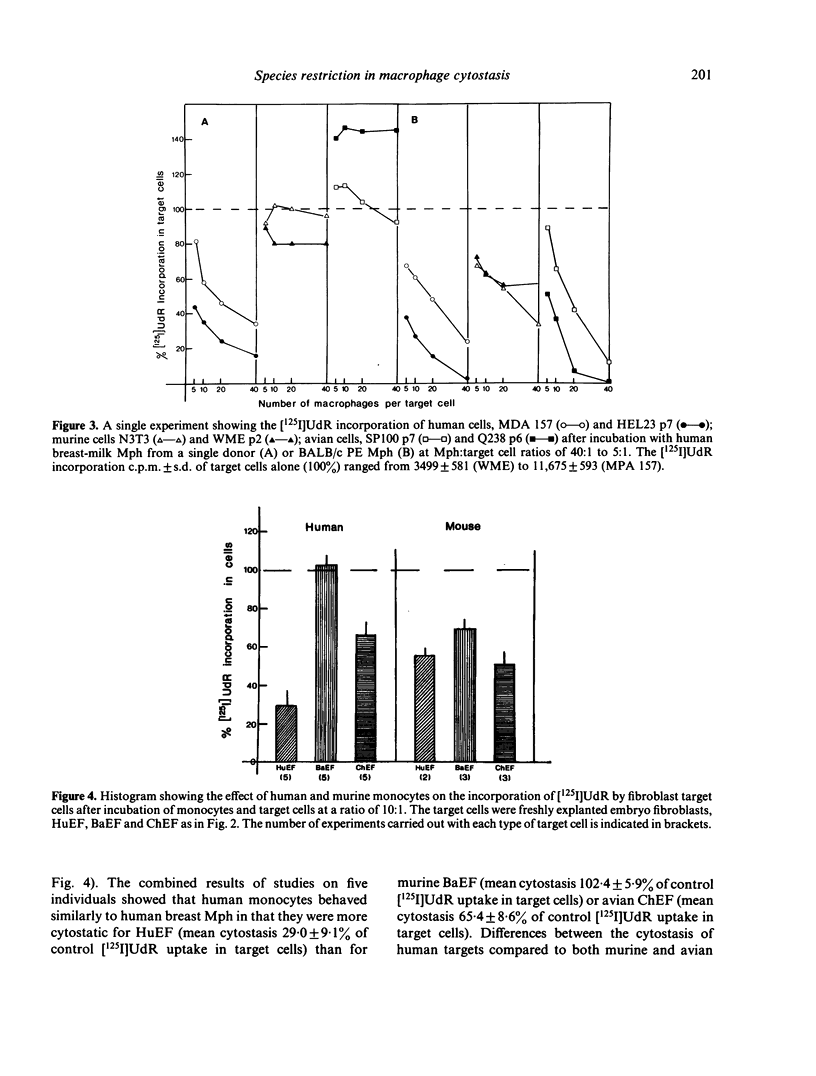
The pattern of species restriction in cytostatic activity of human breast-milk macrophages (Mph) and murine starch-activated peritoneal exudate (PE) Mph was investigated. Human Mph had appreciable cytostatic activity only for human target cells and not for murine or avian target cells. In contrast, murine Mph were particularly cytostatic for target cells from heterologous species and not as cytostatic for other murine cells. This difference in the activity of murine Mph was more notable when freshly explanted fibroblasts were used as target cells than when the cytostasis of long-term tissue culture lines was measured. Experiments with peripheral blood monocytes from the two species indicated that this pattern of reactivity may be common to mononuclear phagocytes from other sources. Therefore, human Mph are preferentially cytostatic for target cells of self species; whereas, murine Mph are equally if not more cytostatic for target cells from other species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Adams D. O., Snyderman R. Do macrophages destroy nascent tumors? J Natl Cancer Inst. 1979 Jun;62(6):1341–1345. [PubMed] [Google Scholar]
- Balkwill F. R., Hogg N. Characterization of human breast milk macrophages cytostatic for human cell lines. J Immunol. 1979 Oct;123(4):1451–1456. [PubMed] [Google Scholar]
- Cabilly S., Gallily R. Studies on the recognition of xenogeneic cells by nonimmune macrophages. I. Destruction of chicken fibroblasts in vitro by murine macrophages. Cell Immunol. 1977 Mar 1;29(1):54–65. doi: 10.1016/0008-8749(77)90274-x. [DOI] [PubMed] [Google Scholar]
- Chang Y. C., Yao C. S. Investigation of the human macrophage. II. The in vitro cytotoxicity of macrophages. Eur J Immunol. 1979 Jul;9(7):521–525. doi: 10.1002/eji.1830090707. [DOI] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature. 1974 Aug 23;250(5468):667–669. doi: 10.1038/250667a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Rosenthal A., Erb P. Macrophage-lymphocyte interactions in immune induction. Int Rev Cytol. 1979;60:149–178. doi: 10.1016/s0074-7696(08)61262-0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hogg N., Parish C. R. Surface antigens of the murine cytostatic peritoneal macrophage. Immunology. 1980 Sep;41(1):187–193. [PMC free article] [PubMed] [Google Scholar]
- Keller R. Susceptibility of normal and transformed cell lines to cytostatic and cytocidal effects exerted by macrophages. J Natl Cancer Inst. 1976 Feb;56(2):369–374. doi: 10.1093/jnci/56.2.369. [DOI] [PubMed] [Google Scholar]
- Lackie A. M. Cellular recognition of foreign-ness in two insect species, the American cockroach and the desert locust. Immunology. 1979 Apr;36(4):909–914. [PMC free article] [PubMed] [Google Scholar]
- Nunn M. E., Djeu J. Y., Glaser M., Lavrin D. H., Herberman R. B. Natural cytotoxic reactivity of rat lymphocytes against syngeneic Gross virus-induced lymphoma. J Natl Cancer Inst. 1976 Feb;56(2):393–399. doi: 10.1093/jnci/56.2.393. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B. Natural cytotoxicity of mouse, rat, and human lymphocytes against heterologous target cells. J Natl Cancer Inst. 1979 Apr;62(4):765–771. [PubMed] [Google Scholar]
- Rinehart J. J., Lange P., Gormus B. J., Kaplan M. E. Human monocyte-induced tumor cell cytotoxicity. Blood. 1978 Jul;52(1):211–220. [PubMed] [Google Scholar]
- Shellam G. R., Hogg N. Gross-virus-induced lymphoma in the rat. IV. Cytotoxic cells in normal rats. Int J Cancer. 1977 Feb 15;19(2):212–224. doi: 10.1002/ijc.2910190211. [DOI] [PubMed] [Google Scholar]
- Sminia T., Borghart-Reinders E., van de Linde A. W. Encapsulation of foreign materials experimentally introduced into the freshwater snail Lymnaea stagnalis. An electron microscopic and autoradiographic study. Cell Tissue Res. 1974;153(3):307–326. doi: 10.1007/BF00229161. [DOI] [PubMed] [Google Scholar]
- Somers S. D., Zwilling B. S. Role of the murine major histocompatibility complex in macrophage-mediated cytolysis. J Immunol. 1978 Dec;121(6):2453–2457. [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Thorpe W. P., Parker G. A., Rosenberg S. A. Expression of fetal antigens by normal human skin cells grown in tissue culture. J Immunol. 1977 Sep;119(3):818–823. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


