Abstract
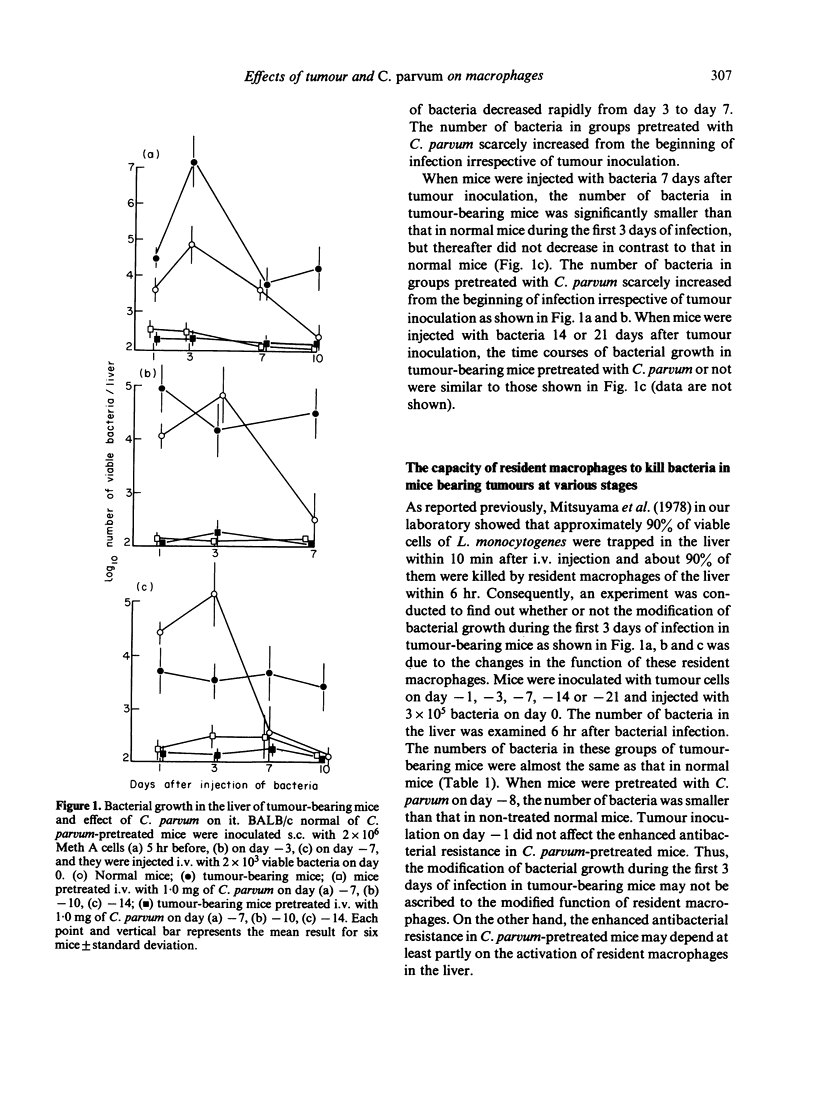
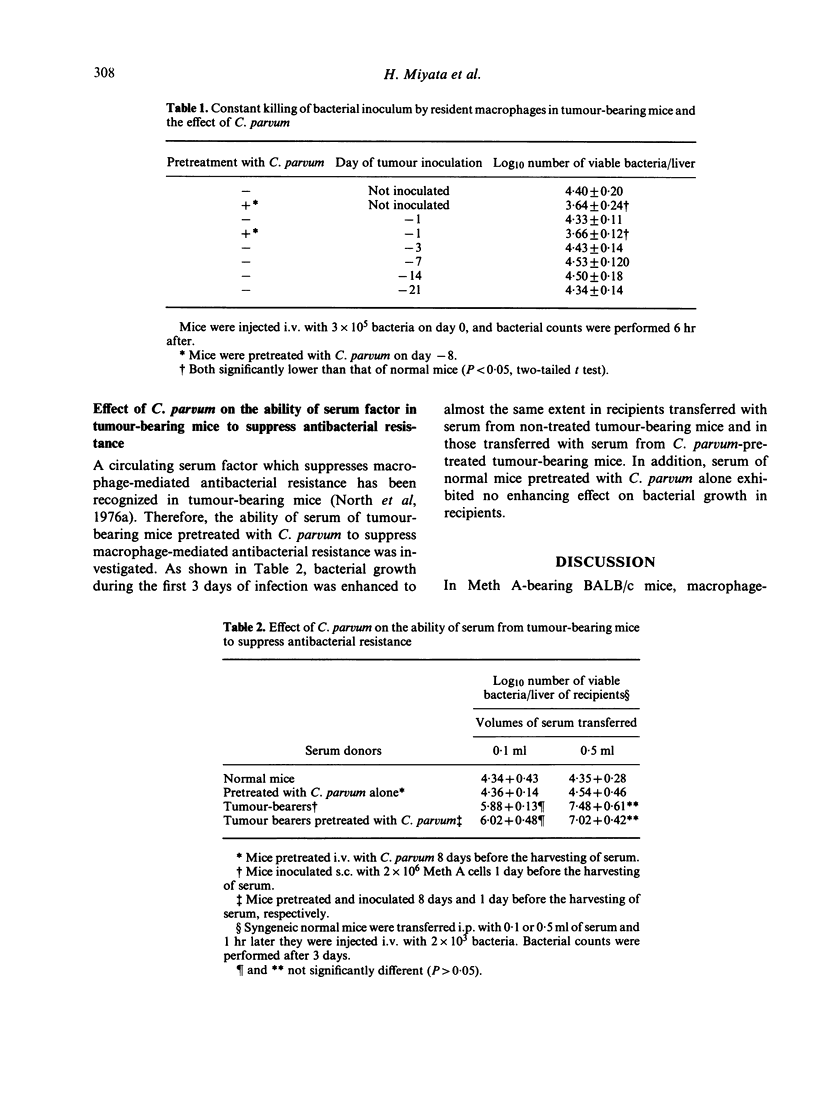
In tumour (fibrosarcoma)-inoculated mice, alterations of host resistance to Listeria monocytogenes following tumour growth were examined. Non-immune macrophage-mediated antibacterial resistance was severely suppressed up to day 4 or so after tumour inoculation, but was enhanced thereafter. On the other hand, T cell-mediated immune resistance retained the control level up to day 7 or so, but was suppressed thereafter. Suppression of macrophage-mediated antibacterial resistance was not observed if the tumour-bearing mice had been pretreated with Corynebacterium parvum. Moreover, this suppression of macrophage-mediated resistance was attributable to the presence of a serum factor that interferes with the function of free macrophages but not with that of resident macrophages. The ability of this serum factor to suppress macrophage-mediated antibacterial resistance, however, was not reduced by C. parvum administration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein I. D., Zbar B., Rapp H. J. Impaired inflammatory response in tumor-bearing guinea pigs. J Natl Cancer Inst. 1972 Dec;49(6):1641–1647. doi: 10.1093/jnci/49.6.1641. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Stevenson M. M. Macrophage function in tumor-bearing mice: tumoricidal and chemotactic responses of macrophages activated by infection with Mycobacterium bovis, strain BCG. J Immunol. 1977 Jun;118(6):2176–2181. [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Normann S. J., Sorkin E. Inhibition of macrophage chemotaxis by neoplastic and other rapidly proliferating cells in vitro. Cancer Res. 1977 Mar;37(3):705–711. [PubMed] [Google Scholar]
- North R. J., Kirstein D. P., Tuttle R. L. Subversion of host defense mechanisms by murine tumors. I. A circulating factor that suppresses macrophage-mediated resistance to infection. J Exp Med. 1976 Mar 1;143(3):559–573. doi: 10.1084/jem.143.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P., Tuttle R. L. Subversion of host defense mechanisms by murine tumors. II. Counter-influence of concomitant antitumor immunity. J Exp Med. 1976 Mar 1;143(3):574–584. doi: 10.1084/jem.143.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a merchanism for abrogation of immune surveillance. J Immunol. 1976 Oct;117(4):1243–1249. [PubMed] [Google Scholar]
- Sher N. A., Poplack D. G., Blaese R. M., Brown T. M., Chaparas S. D. Effect of Corynebacterium parvum, methanol-extraction residue of BCG, and levamisole on macrophage random migration, chemotaxis, and pinocytosis. J Natl Cancer Inst. 1977 Jun;58(6):1753–1757. doi: 10.1093/jnci/58.6.1753. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. An inhibitor of macrophage chemotaxis produced by neoplasms. Science. 1976 Apr 23;192(4237):370–372. doi: 10.1126/science.946556. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Meltzer M. S. Depressed chemotactic responses in vitro of peritoneal macrophages from tumor-bearing mice. J Natl Cancer Inst. 1976 Oct;57(4):847–852. doi: 10.1093/jnci/57.4.847. [DOI] [PubMed] [Google Scholar]


