Abstract
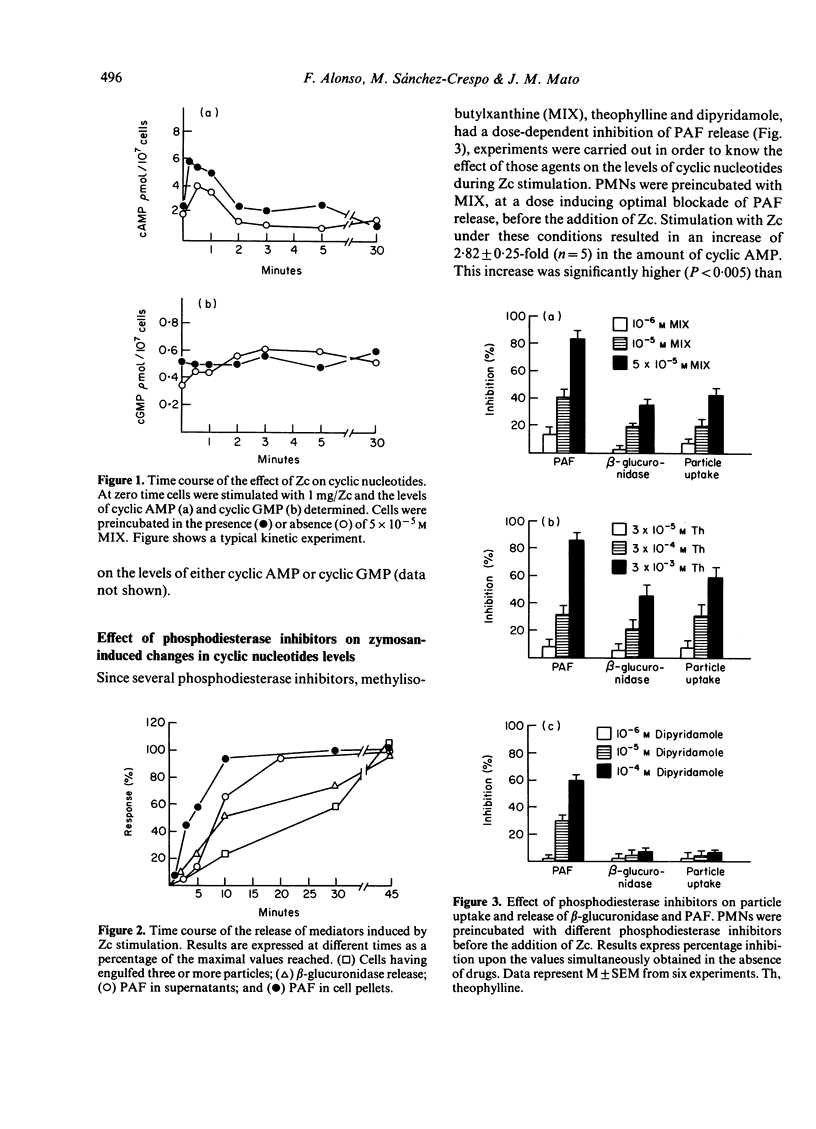
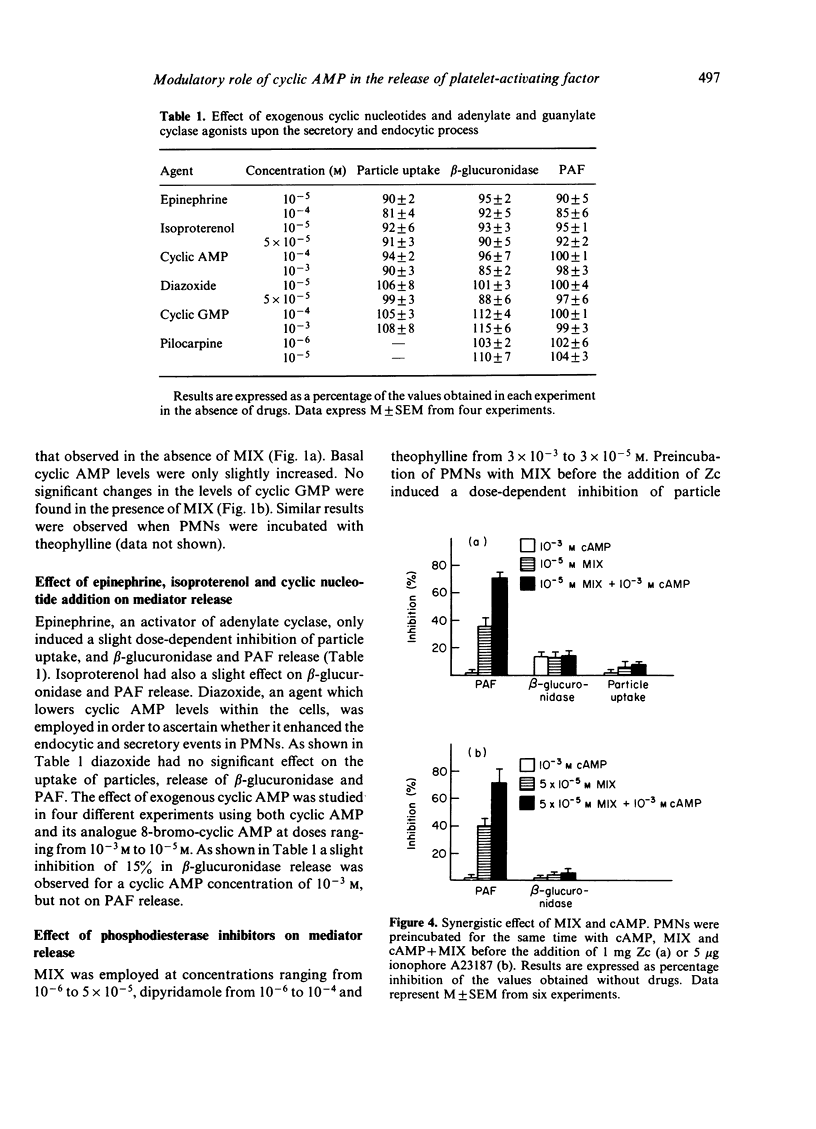
Zymosan coated with complement (Zc) was observed to induce a transient elevation of the intracellular cyclic AMP in human polymorphonuclear cells: a two- to three-fold increase was observed within 1 min after stimulation and approached prestimulation levels by 2 min incubation. These changes in cyclic AMP were not associated with significant changes in cyclic GMP levels. Zymosan caused the release of PAF and beta-glucuronidase and particle uptake, which was initiated about 5 min after stimulation. These results suggest that the transient increase in cyclic AMP content might regulate an early event during mediator release. In an attempt to study further the significance of this rise in cyclic AMP, cells were preincubated with various phosphodiesterase inhibitors. Preincubation of the cells with methylisobutylxanthine (MIX, 10(-6) M to 5 X 10(-5) M), theophylline (3 X 10(-5) to 3 X 10(-3) M) or dipyridamole (10(-6) M to 10(-4) M) enhanced the increase in cyclic AMP levels, but resulted in dose-dependent inhibition of Zc-induced mediator release. Particle uptake and beta-glucuronidase release were less sensitive than PAF release to phosphodiesterase inhibitors, which argues in favour of the independence of both phenomena. Synergistic experiments with MIX and cyclic AMP indicate that the effect of this drug is through its action on cyclic AMP levels. These results suggest that while Zc-induced cyclic AMP elevation might occur in an intracellular place critical to its effect; phosphodiesterase inhibitors may elevate cyclic AMP levels throughout the cell and therefore inhibit the biological response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz S. J., Lotner G. Z., Henson P. M. Generation and release of platelet-activating factor (PAF) from enriched preparations of rabbit basophils; failure of human basophils to release PAF. J Immunol. 1980 Dec;125(6):2749–2755. [PubMed] [Google Scholar]
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Benveniste J. Pharmacological modulation of platelet-activating factor (PAF) release from rabbit leucocytes. I. Role of cAMP. Immunology. 1980 Jul;40(3):367–376. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Caligaris Cappio F., Coda R., Masera C., Segoloni G. Mediators of immune-complex-induced aggregation of polymorphonuclear neutrophils. II. Platelet-activating factor as the effector substance of immune-induced aggregation. Int Arch Allergy Appl Immunol. 1981;64(1):25–41. doi: 10.1159/000232671. [DOI] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Egido J., Alonso F., Sanchez Crespo M., Barat A., Hernando L. Absence of an anaphylactic vasopermeability mechanism for immune complex deposition in the Heymann nephritis of rats. Clin Exp Immunol. 1980 Oct;42(1):99–106. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Derian C. K., Tauber A. I., Valone F. H. Novel effects of 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphorycholine mediators on human leukocyte function: delineation of the specific roles of the acyl substituents. Biochem Biophys Res Commun. 1980 Jun 16;94(3):881–888. doi: 10.1016/0006-291x(80)91317-0. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Lint T. F., George W. J. Hormonal control of lysosomal enzyme release from human neutrophils. Effects of autonomic agents on enzyme release, phagocytosis, and cylic nucleotide levels. J Exp Med. 1974 Jun 1;139(6):1395–1414. doi: 10.1084/jem.139.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmeyer J. E., Johnston R. B., Jr Effect of anti-inflammatory drugs and agents that elevate intracellular cyclic AMP on the release of toxic oxygen metabolites by phagocytes: studies in a model of tissue-bound IgG. Clin Immunol Immunopathol. 1978 Apr;9(4):482–490. doi: 10.1016/0090-1229(78)90144-7. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- Lotner G. Z., Lynch J. M., Betz S. J., Henson P. M. Human neutrophil-derived platelet activating factor. J Immunol. 1980 Feb;124(2):676–684. [PubMed] [Google Scholar]
- Marone G., Thomas L. L., Lichtenstein L. M. The role of agonists that activate adenylate cyclase in the control of cAMP metabolism and enzyme release by human polymorphonuclear leukocytes. J Immunol. 1980 Nov;125(5):2277–2283. [PubMed] [Google Scholar]
- Pryzwansky K. B., Steiner A. L., Spitznagel J. K., Kapoor C. L. Compartmentalization of cyclic AMP during phagocytosis by human neutrophilic granulocytes. Science. 1981 Jan 23;211(4480):407–410. doi: 10.1126/science.6261328. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Smith P. A., Weight F. F., Lehne R. A. Potentiation of Ca2+-dependent K+ activation by theophylline is independent of cyclic nucleotide elevation. Nature. 1979 Aug 2;280(5721):400–402. doi: 10.1038/280400a0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Sánchez-Crespo M., Alonso F., Egido J. Platelet-activating factor in anaphylaxis and phagocytosis. I. Release from human peripheral polymorphonuclears and monocytes during the stimulation by ionophore A23187 and phagocytosis but not from degranulating basophils. Immunology. 1980 Aug;40(4):645–655. [PMC free article] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]


