Abstract
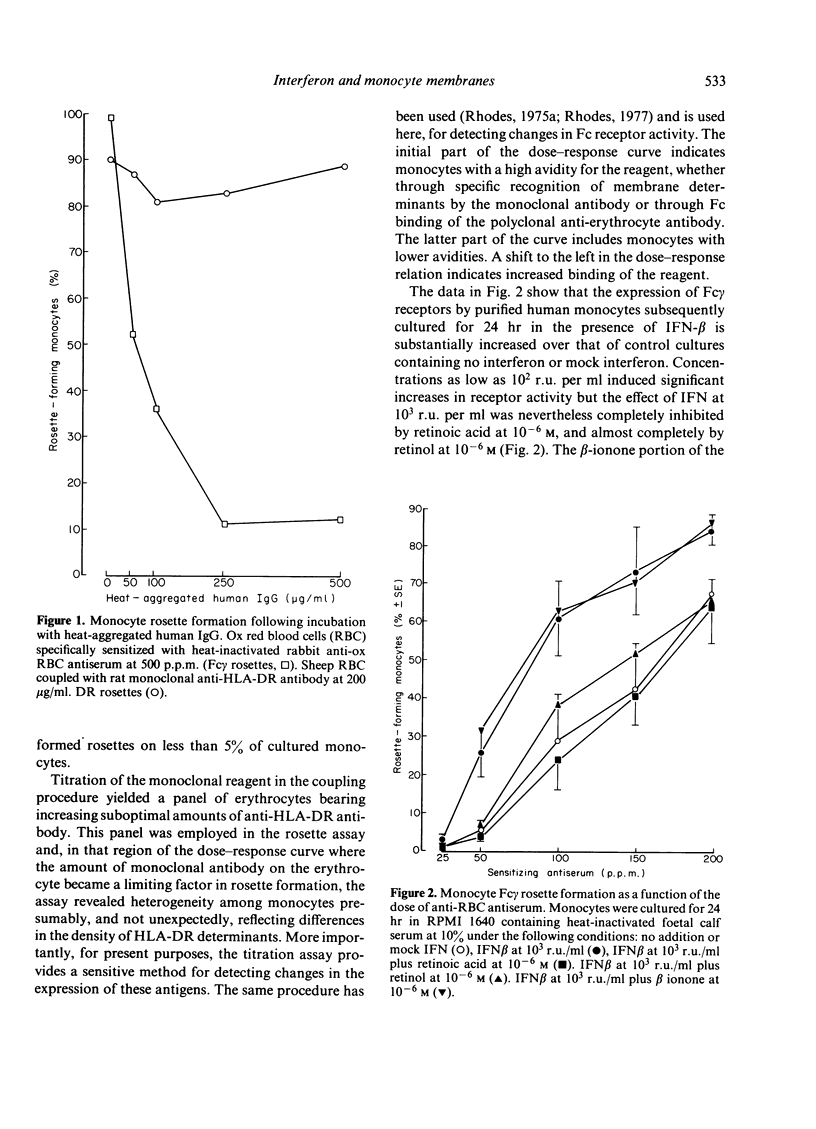
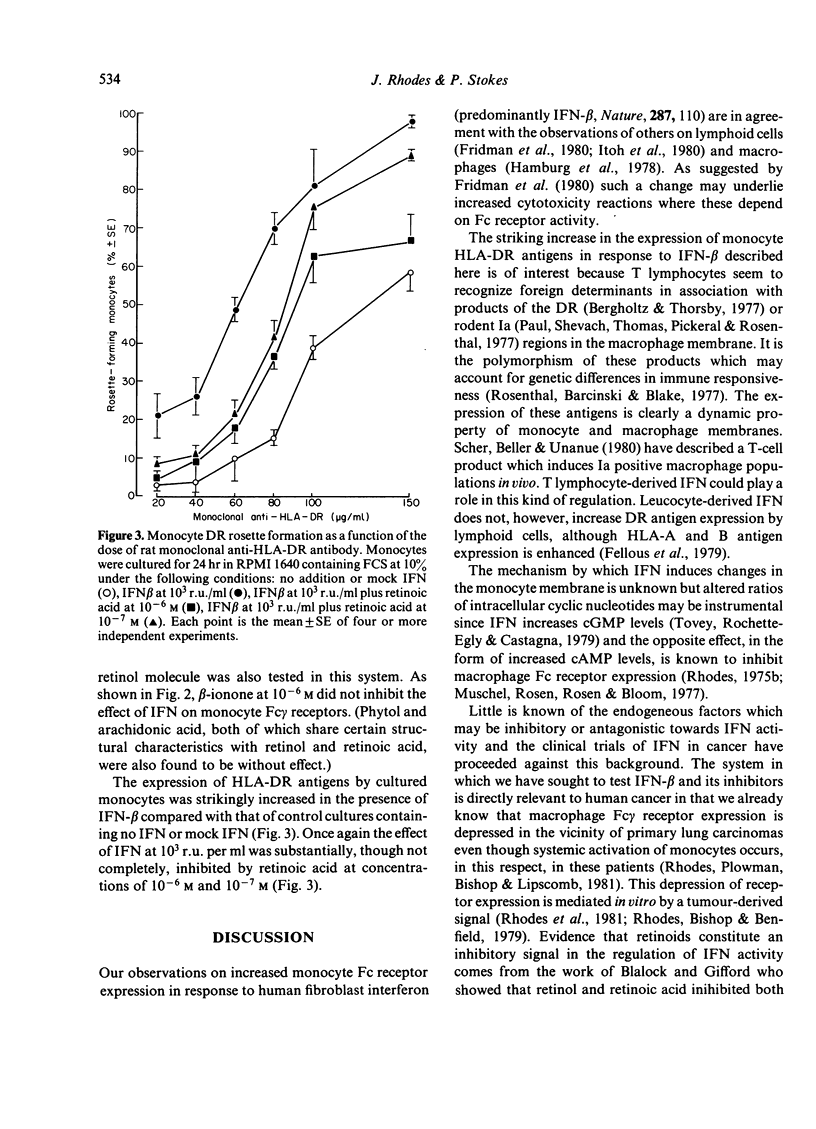
The effects of human interferon-beta on human peripheral blood monocyte function were examined in vitro. Interferon-beta was shown to increase substantially the expression of both Fc gamma receptors and HLA-DR antigens defined by monoclonal antibody. Retinol and retinoic acid were found to be antagonistic to these effects of interferon and physiological concentrations were sufficient to inhibit changes in the monocyte membrane induced by relatively high concentrations of interferon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergholtz B. O., Thorsby E. Macrophage-dependent response of immune human T lymphocytes to PPD in vitro. Influence of HLA-D histocompatibility. Scand J Immunol. 1977;6(8):779–786. doi: 10.1111/j.1365-3083.1977.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Gifford G. E. Inhibition of interferon action by vitamin A. J Gen Virol. 1975 Dec;29(3):315–324. doi: 10.1099/0022-1317-29-3-315. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Gifford G. E. Retinoic acid (vitamin A acid) induced transcriptional control of interferon production. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5382–5386. doi: 10.1073/pnas.74.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., McConnell I., Milstein C., Wright B. A monoclonal antibody to the HLA-DR product recognizes a polymorphic Ia determinant in mice. Immunology. 1981 Jul;43(3):493–501. [PMC free article] [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular vitamin A binding proteins. Vitam Horm. 1978;36:1–32. doi: 10.1016/s0083-6729(08)60980-2. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Wilson A. B., Eremin O., Gurner B. W., Haegert D. G., Lawson Y. A., Bright S., Munro A. J. Comparison of the direct antiglobulin rosetting reaction with the mixed antiglobulin rosetting reaction for the detection of immunoglobulin on lymphocytes. J Immunol Methods. 1977;18(1-2):45–54. doi: 10.1016/0022-1759(77)90157-0. [DOI] [PubMed] [Google Scholar]
- Fellous M., Kamoun M., Gresser I., Bono R. Enhanced expression of HLA antigens and beta 2-microglobulin on interferon-treated human lymphoid cells. Eur J Immunol. 1979 Jun;9(6):446–449. doi: 10.1002/eji.1830090606. [DOI] [PubMed] [Google Scholar]
- Fridman W. H., Gresser I., Bandu M. T., Aguet M., Neauport-Sautes C. Interferon enhances the expression of Fc gamma receptors. J Immunol. 1980 May;124(5):2436–2441. [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Hamburg S. I., Manejias R. E., Rabinovitch M. Macrophage activation: increased ingestion of IgG-coated erythrocytes after administration of interferon inducers to mice. J Exp Med. 1978 Feb 1;147(2):593–598. doi: 10.1084/jem.147.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Itoh K., Inoue M., Kataoka S., Kumagai K. Differential effect of interferon expression of IgG- and IgM-Fc receptors on human lymphocytes. J Immunol. 1980 Jun;124(6):2589–2595. [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the specific cytotoxicity of sensitized lymphocytes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):721–725. doi: 10.1073/pnas.69.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Dean J. H., Jerrells T. R., Herberman R. B. Augmentation of tumoricidal activity of human monocytes and macrophages by lymphokines. Int J Cancer. 1980 Jun 15;25(6):691–699. doi: 10.1002/ijc.2910250602. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Rosen O. M., Bloom B. R. Modulation of Fc-mediated phagocytosis by cyclic AMP and insulin in a macrophage-like cell line. J Immunol. 1977 Nov;119(5):1813–1820. [PubMed] [Google Scholar]
- Paul W. E., Shevach E. M., Thomas D. W., Pickeral S. F., Rosenthal A. S. Genetic restriction in T-lymphocyte activation by antigen-pulse peritoneal exudate cells. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):571–578. doi: 10.1101/sqb.1977.041.01.066. [DOI] [PubMed] [Google Scholar]
- Peto R., Doll R., Buckley J. D., Sporn M. B. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981 Mar 19;290(5803):201–208. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Altered expression of human monocyte Fc receptors in malignant disease. Nature. 1977 Jan 20;265(5591):253–255. doi: 10.1038/265253a0. [DOI] [PubMed] [Google Scholar]
- Rhodes J., Bishop M., Benfield J. Tumor surveillance: how tumors may resist macrophage-mediated host defense. Science. 1979 Jan 12;203(4376):179–182. doi: 10.1126/science.758686. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Macrophage heterogeneity in receptor activity: the activation of macrophage Fc receptor function in vivo and in vitro. J Immunol. 1975 Mar;114(3):976–981. [PubMed] [Google Scholar]
- Rhodes J. Modulation of macrophage Fc receptor expression in vitro by insulin and cyclic nucleotides. Nature. 1975 Oct 16;257(5527):597–599. doi: 10.1038/257597a0. [DOI] [PubMed] [Google Scholar]
- Rhodes J., Oliver S. Retinoids as regulators of macrophage function. Immunology. 1980 Jul;40(3):467–472. [PMC free article] [PubMed] [Google Scholar]
- Rhodes J., Plowman P., Bishop M., Lipscomb D. Human macrophage function in cancer: systemic and local changes detected by an assay for Fc receptor expression. J Natl Cancer Inst. 1981 Mar;66(3):423–429. [PubMed] [Google Scholar]
- Rosenthal A. S., Barcinski M. A., Blake J. T. Determinant selection is a macrophage dependent immune response gene function. Nature. 1977 May 12;267(5607):156–158. doi: 10.1038/267156a0. [DOI] [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Papamatheakis J. D., Chirigos M. A. Interferon: an inducer of macrophage activation by polyanions. Science. 1977 Aug 12;197(4304):674–676. doi: 10.1126/science.877584. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Rochette-Egly C., Castagna M. Effect of interferon on concentrations of cyclic nucleotides in cultured cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3890–3893. doi: 10.1073/pnas.76.8.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Karre K., Hansson M., Kunkel L. A., Kiessling R. W. Interferon-mediated protection of normal and tumor target cells against lysis by mouse natural killer cells. J Immunol. 1981 Jan;126(1):219–225. [PubMed] [Google Scholar]


