Abstract
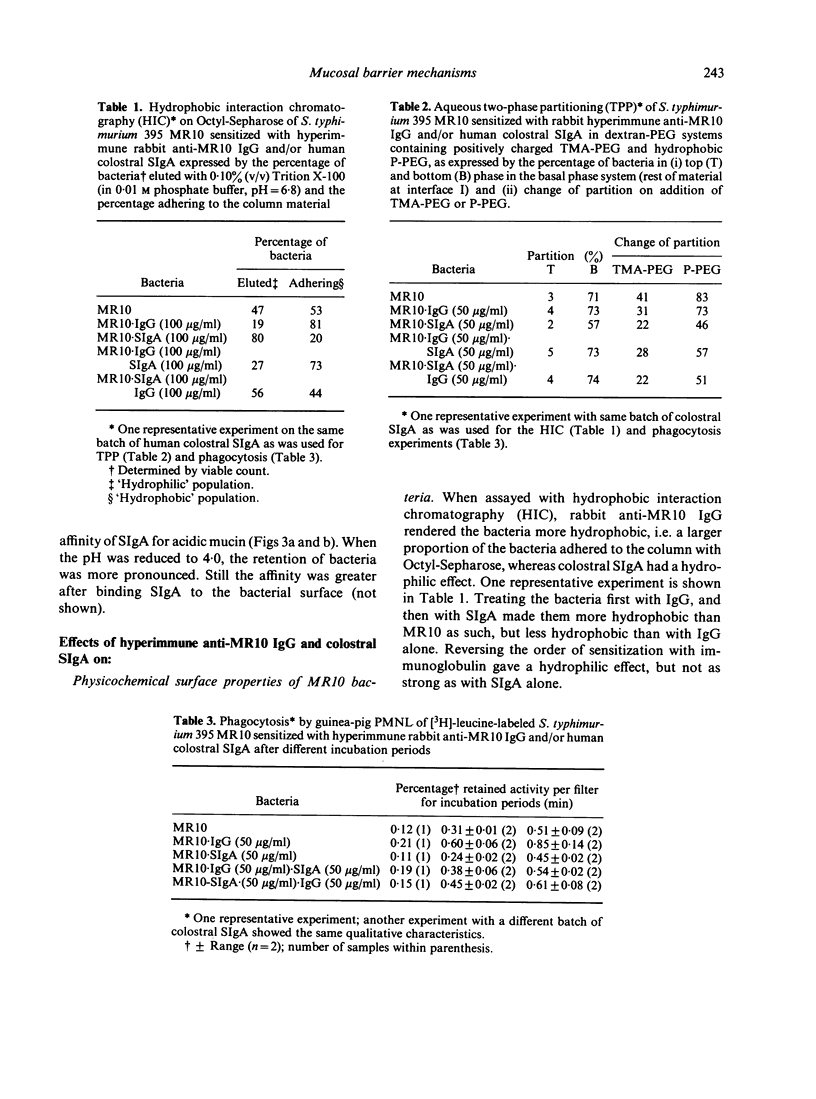
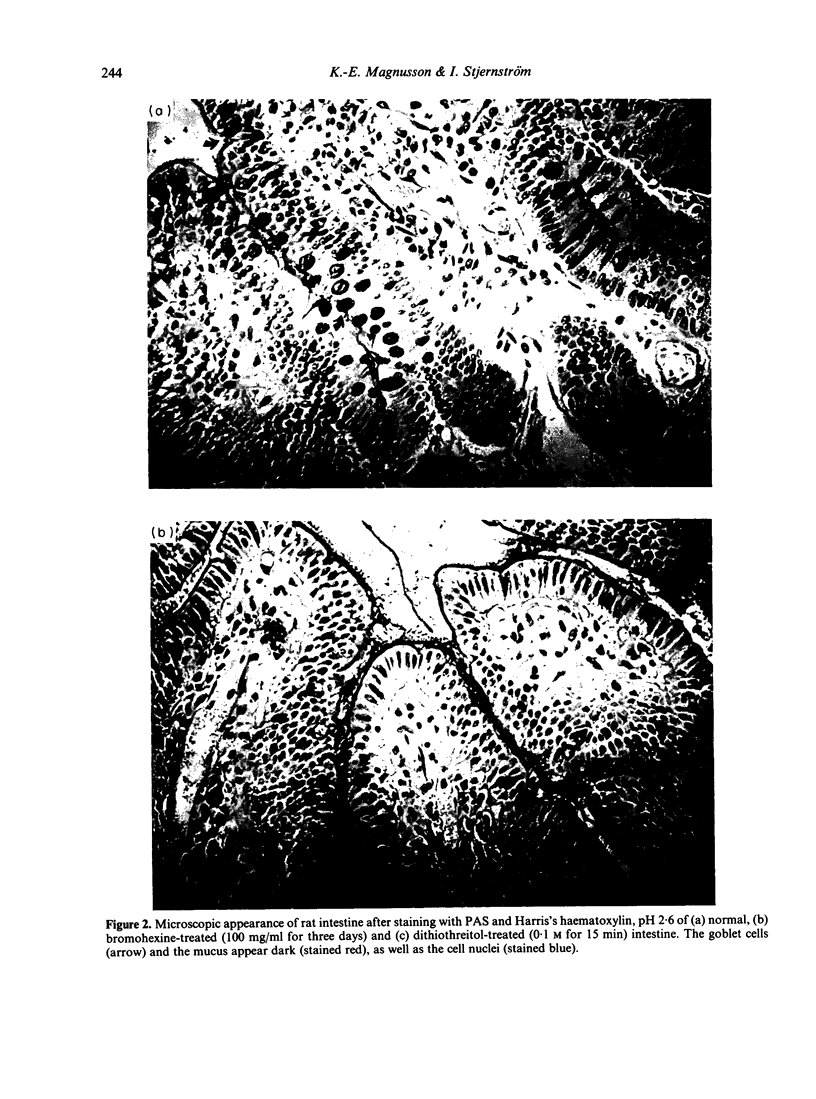
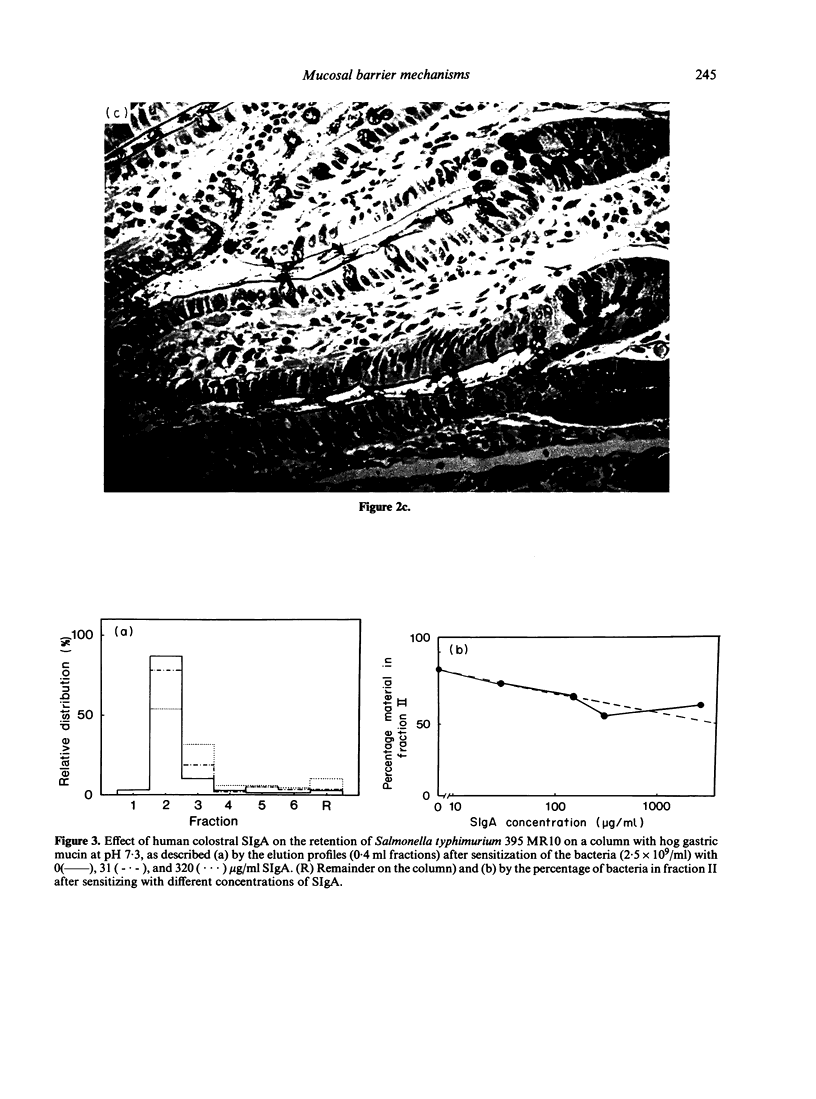
Rough Salmonella typhimurium 395 MR 10 bacteria sensitized with SIgA were used to assess the effect of secretory IgA (SIgA) on bacterial association with the intestine of rat and with a column of hog gastric mucin, and on IgG-mediated surface properties and interaction with polymorphonuclear leucocytes. It was found that SIgA increased the affinity for mucus belt of the intestine and for the mucin column, but reduced IgG-enhanced phagocytosis and surface hydrophobicity and charge of the bacteria. It is suggested that the ability of SIgA to render bacteria mucophilic and to modify IgG-mediated reactions serve the purpose of secluding bacteria from contact with mucosal membranes and depress inflammatory reactions at the site of infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P., Tolo K. Mucosal penetrability enhanced by serum-derived antibodies. Nature. 1977 Mar 17;266(5599):262–263. doi: 10.1038/266262a0. [DOI] [PubMed] [Google Scholar]
- Bruce R. A., Kumar V. The effect of a derivative of vasicine on bronchial mucus. Br J Clin Pract. 1968 Jul;22(7):289–292. [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Etzler M. E. Lectins as probes in studies of intestinal glycoproteins and glycolipids. Am J Clin Nutr. 1979 Jan;32(1):133–138. doi: 10.1093/ajcn/32.1.133. [DOI] [PubMed] [Google Scholar]
- FLOREY H. W. The secretion and function of intestinal mucus. Gastroenterology. 1962 Sep;43:326–329. [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- HEATLEY N. G. Mucosubstance as a barrier to diffusion. Gastroenterology. 1959 Sep;37:313–317. [PubMed] [Google Scholar]
- Johansson G. Studies on aqueous dextran-poly (ethylene glycol) two-phase systems containing charged poly (ethylene glycol). I. Partition of albumins. Biochim Biophys Acta. 1970 Nov 24;222(2):381–389. doi: 10.1016/0304-4165(70)90127-3. [DOI] [PubMed] [Google Scholar]
- Johansson G. The effect of poly(ethyleneglycol) esters on the partition of proteins and fragmented membranes in aqueous biphasic systems. Biochim Biophys Acta. 1976 Dec 21;451(2):517–529. doi: 10.1016/0304-4165(76)90147-1. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Magnusson K. E. Association with HeLa cells of LPS mutants of Salmonella typhimurium and Salmonella minnesota in relation to their physicochemical surface properties. Cell Biophys. 1980 Sep;2(3):177–189. doi: 10.1007/BF02790448. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Stendahl O., Stjernström I., Edebo L. Reduction of phagocytosis, surface hydrophobicity and charge of Salmonella typhimurium 395 MR10 by reaction with secretory IgA (SIgA). Immunology. 1979 Mar;36(3):439–447. [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Baetz A. L. Comparative effects of enterotoxins from Escherichia coli and Vibrio cholerae on rabbit and swine small intestine. Lab Invest. 1971 Aug;25(2):133–140. [PubMed] [Google Scholar]
- Steinberg S. E., Banwell J. G., Yardley J. H., Keusch G. T., Hendrix T. R. Comparison of secretory and histological effects of shigella and cholera enterotoxins in rabbit jejunum. Gastroenterology. 1975 Feb;68(2):309–317. [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Edebo M. Partition of Salmonella typhimurium in a two-polymer acqueous phase system in relation to liability to phagocytosis. Infect Immun. 1973 Jul;8(1):36–41. doi: 10.1128/iai.8.1.36-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernström I., Magnusson K. E., Stendahl O., Tagesson C. Liability to hydrophobic and charge interaction of smooth Salmonella typhimurium 395 MS sensitized with anti-MS immunoglobulin G and complement. Infect Immun. 1977 Nov;18(2):261–265. doi: 10.1128/iai.18.2.261-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C. R., Soothill J. F., Turner M. W. Immune exclusion is a function of IgA. Nature. 1975 Jun 26;255(5511):745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Wu M., Bloch K. J. Stimulation by immune complexes of mucus release from goblet cells of the rat small intestine. Science. 1977 Jul 22;197(4301):370–372. doi: 10.1126/science.877559. [DOI] [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- Wilton J. M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978 Dec;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]




