Abstract
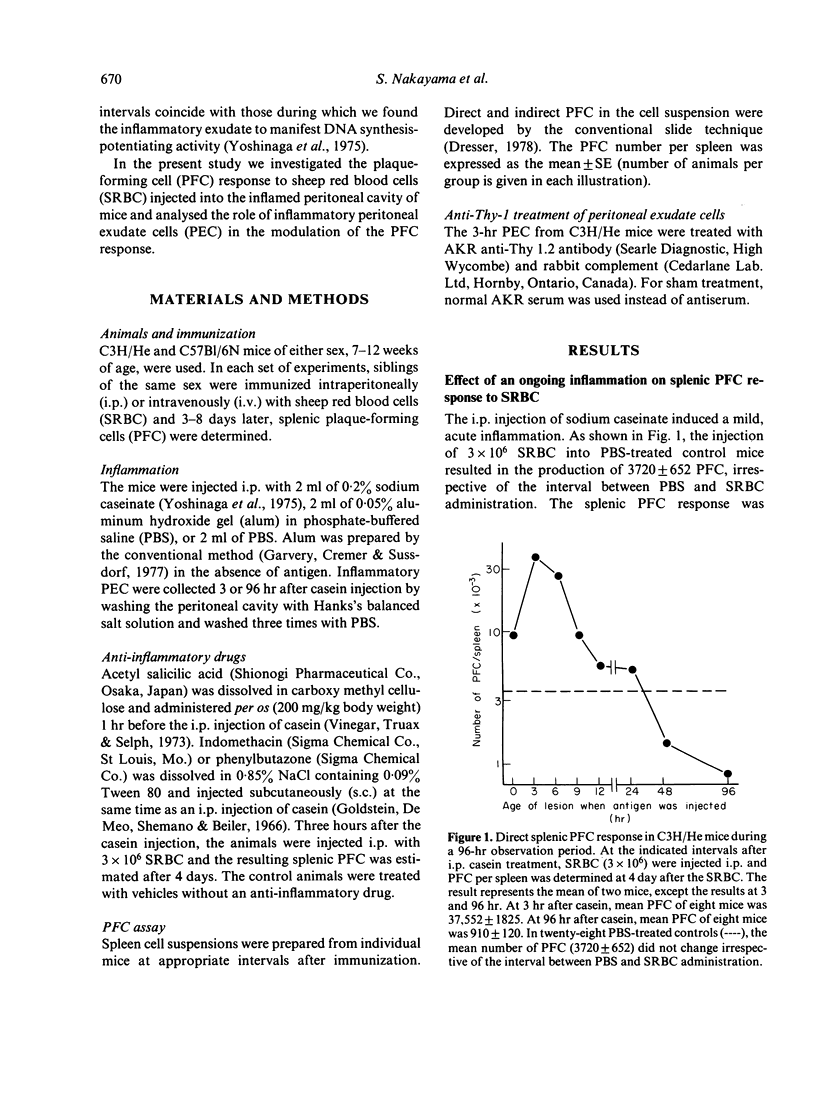
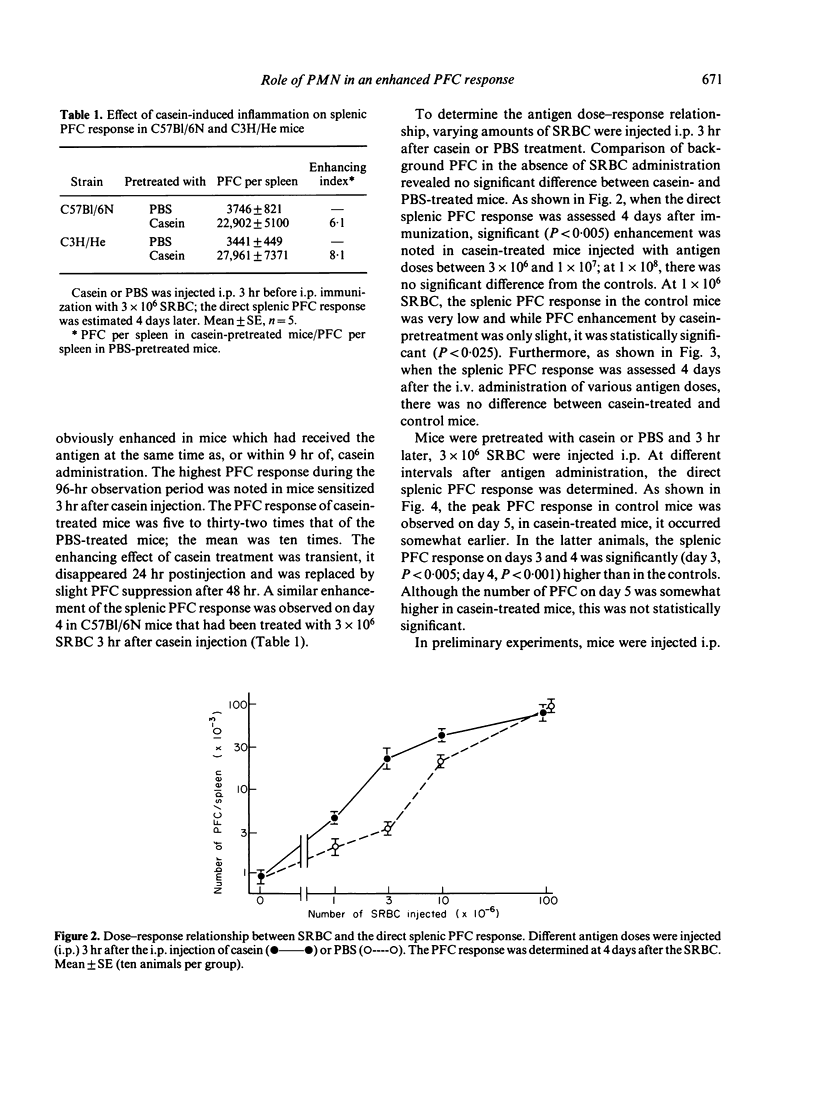
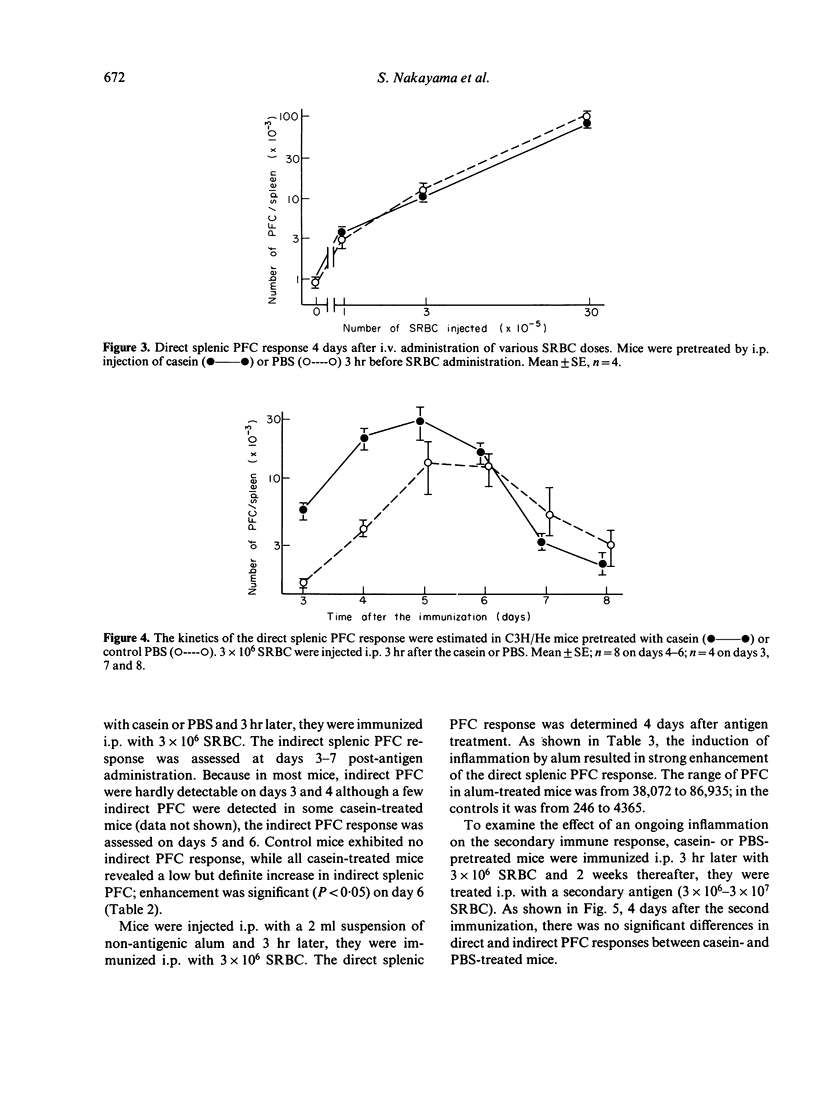
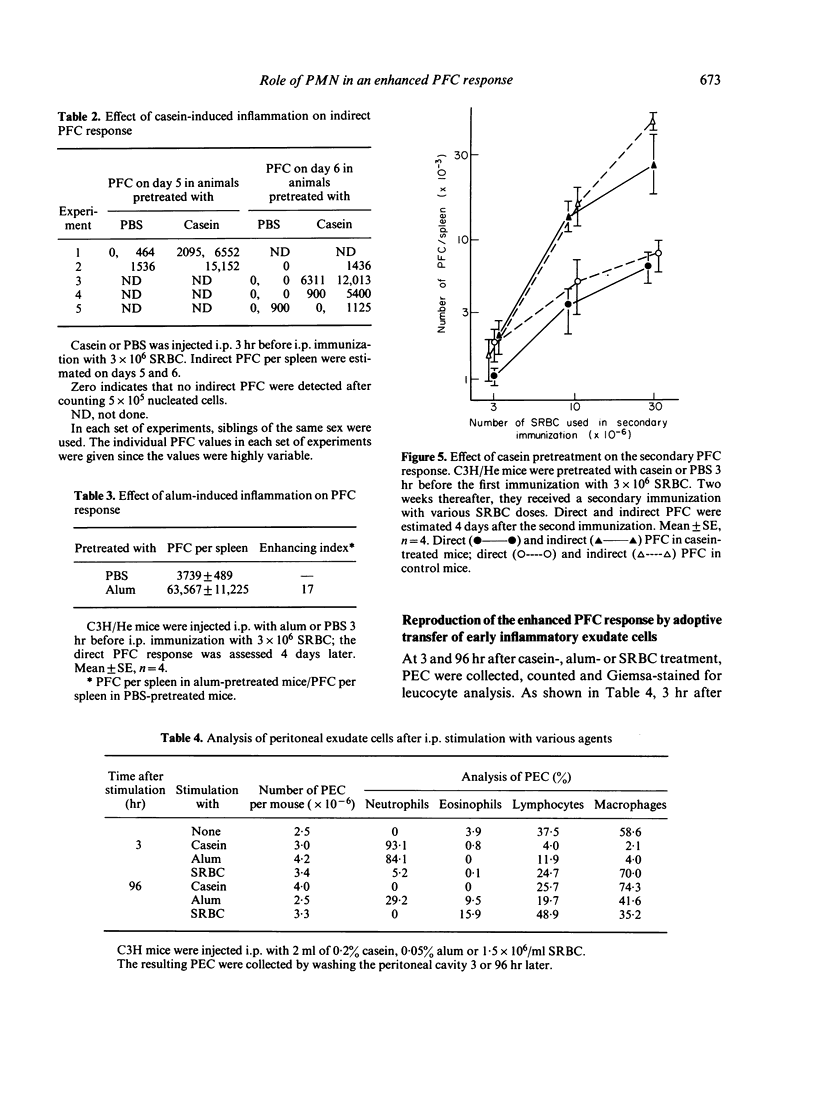
The effect of inflammation induced by sodium caseinate or aluminum hydroxide on the splenic plaque-forming cells (PFC) response to sheep red blood cells (SRBC) was studied in mice. Direct and indirect splenic PFC responses were enhanced when suboptimal SRBC doses (3 x 10(6)) were injected intraperitoneally (i.p.) within 9 hr of i.p. inflammatory stimulation; antigen administration 48 hr or more after such stimulation resulted in a slight suppression of the direct response. The inflammation had no effect on the secondary immune response, nor did intravenous antigen administration enhance the PFC response. Enhancement occurred when early (3 hr), casein-induced peritoneal exudate cells (PEC, consisting mostly of neutrophils) were adoptively transferred at the same time as antigen. Treatment of the 3-hr PEC with anti-Thy-1 and complement did not decrease their PFC-enhancing capability. Late (96-hr) PEC, consisting mostly of macrophages, manifested only a slight enhancing effect. We suggest that enhancement of the splenic PFC response in the presence of an ongoing inflammation, may be partially attributable to neutrophil function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOEHME D., DUBOS R. J. The effect of bacterial constituents on the resistance of mice to heterologous infection and on the activity of their reticulo-endothelial system. J Exp Med. 1958 Apr 1;107(4):523–536. doi: 10.1084/jem.107.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield J. W., Souhami R. L., Addison I. E. The mechanism of the adjuvant effect of dextran sulphate. Immunology. 1974 Feb;26(2):383–392. [PMC free article] [PubMed] [Google Scholar]
- Braun W., Firshein W. Biodynamic effects of oligonucleotides. Bacteriol Rev. 1967 Jun;31(2):83–94. doi: 10.1128/br.31.2.83-94.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depelchin A., Huygen K. Interference between two immune responses. I.--Modification of the kinetics of the mouse response to SRBC by responses to heterologous RBC. Ann Immunol (Paris) 1977 Apr-Jun;128C(3):633–644. [PubMed] [Google Scholar]
- Di Rosa M., Papadimitriou J. M., Willoughby D. A. A histopathological and pharmacological analysis of the mode of action of nonsteroidal anti-inflammatory drugs. J Pathol. 1971 Dec;105(4):239–256. doi: 10.1002/path.1711050403. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Meinhold H., Wagner B. Stimulation of humoral antibody formation by polyanions. V. Relationship between enhancement of sheep red blood cell uptake by the spleen and adjuvant action of dextran sulfate. Eur J Immunol. 1971 Dec;1(6):429–432. doi: 10.1002/eji.1830010605. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. An assay for adjuvanticity. Clin Exp Immunol. 1968 Nov;3(9):877–888. [PMC free article] [PubMed] [Google Scholar]
- Franzl R. E., McMaster P. D. The primary immune response in mice. I. The enhancement and suppression of hemolysin production by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1087–1107. doi: 10.1084/jem.127.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender M. H., Chisari F. V., Baer H. The role of the inflammatory response of skin and lymph nodes in the induction of sensitization to simple chemicals. J Immunol. 1973 Jul;111(1):164–170. [PubMed] [Google Scholar]
- Ghaffar A., Sigel M. M. Immunomodulation by Corynebacterium parvum. 1. Variable effects on anti-sheep erythrocyte antibody responses. Immunology. 1978 Nov;35(5):685–693. [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., DeMeo R., Shemano I., Beiler J. M. A method for differentiating nonspecific irritants from anti-inflammatory agents using the carrageenin abscess test. Proc Soc Exp Biol Med. 1966 Dec;123(3):712–715. doi: 10.3181/00379727-123-31584. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Scott M. T. Biological effects of Corynebacterium parvum. IV. Adjuvant and inhibitory activities on B lymphocytes. Cell Immunol. 1973 May;7(2):290–301. doi: 10.1016/0008-8749(73)90251-7. [DOI] [PubMed] [Google Scholar]
- Kinnaert P., Mahieu A., van Geertruyden N. Kinetics of humoral responsiveness and antigenic distribution in operated rats. Immunology. 1979 Sep;38(1):195–201. [PMC free article] [PubMed] [Google Scholar]
- LAUFER A., TAL C., BEHAR A. J. Effect of adjuvant (Freund's type) and its components on the organs of various animal species; a comparative study. Br J Exp Pathol. 1959 Feb;40(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Möller G., Sjöberg O. Effect of antigenic competition on antigen-sensitive cells and on adoptively transferred immunocompetent cells. Cell Immunol. 1970 May;1(1):110–121. doi: 10.1016/0008-8749(70)90064-x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Yoshinaga M., Hayashi H. Interaction between lymphocytes and inflammatory exudate cells. II. A proteolytic enzyme released by PMN as a possible mediator for enhancement of thymocyte response. J Immunol. 1976 Jul;117(1):1–6. [PubMed] [Google Scholar]
- PERNIS B., PARONETTO F. Adjuvant effect of silica (tridymite) on antibody production. Proc Soc Exp Biol Med. 1962 Jun;110:390–392. doi: 10.3181/00379727-110-27527. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. The role of opsonins in non-specific immunity. J Exp Med. 1960 Jan 1;111:137–144. doi: 10.1084/jem.111.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sljivić V. S., Clark D. W., Warr G. W. Effects of oestrogens and pregnancy on the distribution of sheep erythrocytes and the antibody response in mice. Clin Exp Immunol. 1975 Apr;20(1):179–186. [PMC free article] [PubMed] [Google Scholar]
- Souhami R. L. The effect of colloidal carbon on the organ distribution of sheep red cells and the immune response. Immunology. 1972 Apr;22(4):685–694. [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., Singer C. R., Tilney N. L., Folch H., MacSween R. N. The immune response in cirrhotic rats. Antigen distribution, humoral immunity, cell-mediated immunity and splenic suppressor cell activity. Clin Exp Immunol. 1976 Dec;26(3):574–582. [PMC free article] [PubMed] [Google Scholar]
- Turner E. V., Higginbotham R. D. Carrageenan induced alterations of antigen distribution and immune responses in mice. J Reticuloendothel Soc. 1977 Dec;22(6):545–554. [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A., Allison A. C. A role of macrophages in the stimulation of immune responses by adjuvants. J Immunol. 1969 Jul;103(1):71–78. [PubMed] [Google Scholar]
- Warr G. W., Sljivić V. S. Enhancement and depression of the antibody response in mice caused by Corynebacterium parvum. Clin Exp Immunol. 1974 Jul;17(3):519–532. [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga M., Nakamura S., Hayashi H. Interaction between lymphocytes and inflammatory exudate cells. I. Enhacement of thymocyte response to PHA by product(s) of polymorphonuclear leukocytes and macrophages. J Immunol. 1975 Aug;115(2):533–538. [PubMed] [Google Scholar]
- Yoshinaga M., Nishime K., Nakamura S., Goto F. A PMN-derived factor that enhances DNA-synthesis in PHA or antigen-stimulated lymphocytes. J Immunol. 1980 Jan;124(1):94–99. [PubMed] [Google Scholar]


