Abstract
We have observed earlier that T lymphocytes of dengue type 2 virus (DV)-infected mouse spleen produce a cytotoxic factor (CF) which kills T lymphocytes and macrophages of the spleen of normal mice or animals of other species. In the present study an effort was made to study the effect of CF treatment on human peripheral blood leucocytes. After treatment with various dilutions of CF at 4 degrees for 1 hr 25%-36% of T lymphocytes lost their capacity to form E rosettes and 25%-36% of monocytes lost their phagocytic function. Cytotoxic-factor treatment had no effect on formation of EAC rosettes by B lymphocytes and the phagocytic functions of polymorphonuclear cells. Pretreatment of cells with 2,4 dinitrophenol, reduced glutathione or ouabain, which act on the cell membrane, inhibited the effect of CF on E-rosette formation and phagocytosis. This indicated that CF acts by inducing changes in the cell membrane. It is likely that production of a similar factor in DV-infected humans is responsible for similar alterations observed in their blood.
Full text
PDF

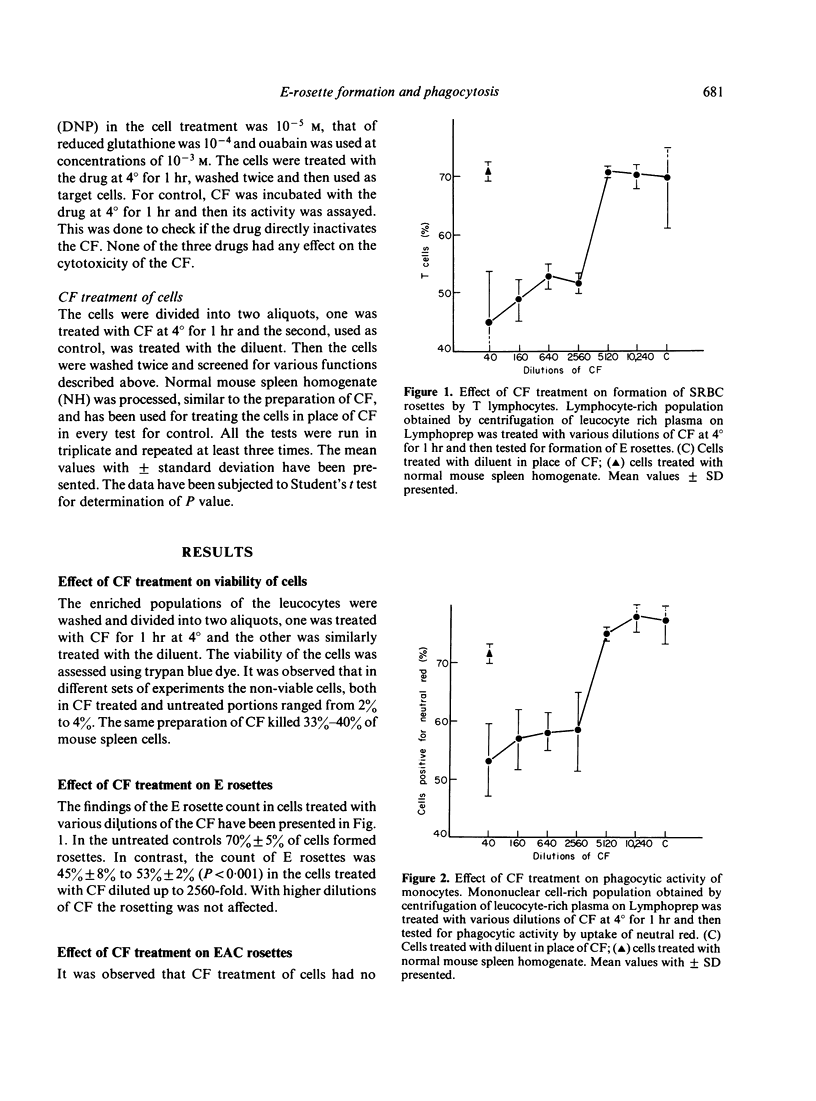
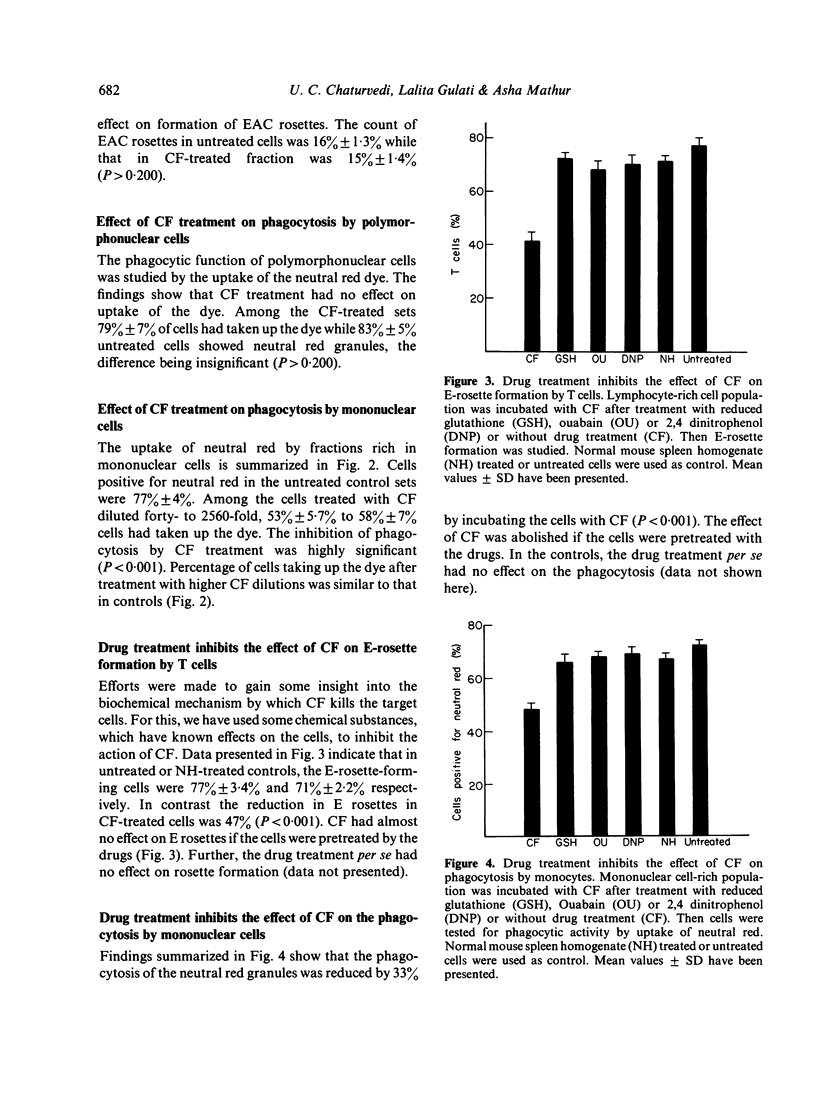



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aung-Khin M., Ma-Ma K., Thant-Zin Changes in the tissues of the immune system in dengue haemorrhagic fever. J Trop Med Hyg. 1975 Dec;78(12):256–261. [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J., Thompson T. E., Lehninger A. L. The effect of 2,4-dinitrophenol on the electrical resistance of phospholipid bilayer membranes. Biochem Biophys Res Commun. 1966 Sep 22;24(6):948–954. doi: 10.1016/0006-291x(66)90342-1. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Bhargava A., Mathur A. Production of cytotoxic factor in the spleen of dengue virus-infected mice. Immunology. 1980 Aug;40(4):665–671. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Dalakoti H., Mathur A. Characterization of the cytotoxic factor produced in the spleen of dengue virus-infected mice. Immunology. 1980 Oct;41(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Mathur A., Tandon P., Natu S. M., Rajvanshi S., Tandon H. O. Variable effect on peripheral blood leucocytes during JE virus infection of man. Clin Exp Immunol. 1979 Dec;38(3):492–498. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon H. O., Mathur A. Control of in vitro and in vivo spread of coxsackievirus B4 infection by sensitized spleen cells and antibody. J Infect Dis. 1978 Aug;138(2):181–190. doi: 10.1093/infdis/138.2.181. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A. Effect of immunosuppression on dengue virus infection in mice. J Gen Virol. 1977 Sep;36(3):449–458. doi: 10.1099/0022-1317-36-3-449. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A., Kumar A. Host defence mechanisms against dengue virus infection of mice. J Gen Virol. 1978 May;39(2):293–302. doi: 10.1099/0022-1317-39-2-293. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Human T lymphocyte "E" rosette function. I. A process modulated by intracellular cyclic AMP. J Exp Med. 1974 Oct 1;140(4):1122–1126. doi: 10.1084/jem.140.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Lymphocyte E rosette inhibitory factor: a regulatory serum lipoprotein. J Exp Med. 1975 Nov 1;142(5):1092–1107. doi: 10.1084/jem.142.5.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER T. N., GINSBERG H. S. The reaction of influenza viruses with guinea pig polymorphonuclear leucocytes. III. Studies on the mechanism by which influenza viruses inhibit phagocytosis. Virology. 1956 Oct;2(5):656–664. doi: 10.1016/0042-6822(56)90045-9. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Remo R. A. Beta-adrenergic inhibition of human T lymphocyte rosettes. J Immunol. 1975 Jan;114(1 Pt 2):512–513. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misefari A., Venza-Teti D., LaVia M. F. Effect of prostaglandins on mouse erythrocyte rosette-forming human lymphocytes. Immunopharmacology. 1980 Apr;2(2):109–115. doi: 10.1016/0162-3109(80)90003-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Nimmannitya S., Halstead S. B., Cohen S. N., Margiotta M. R. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969 Nov;18(6):954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- Venza-Teti D., Misefari A., Sofo V., Fimiani V., LaVia M. F. Interaction between prostaglandins and human T lymphocytes: effect of PGE2 on E-receptor expression. Immunopharmacology. 1980 Apr;2(2):165–171. doi: 10.1016/0162-3109(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Wells R. A., Scott R. M., Pavanand K., Sathitsathein V., Cheamudon U., Macdermott R. P. Kinetics of peripheral blood leukocyte alterations in Thai children with dengue hemorrhagic fever. Infect Immun. 1980 May;28(2):428–433. doi: 10.1128/iai.28.2.428-433.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]


