Abstract
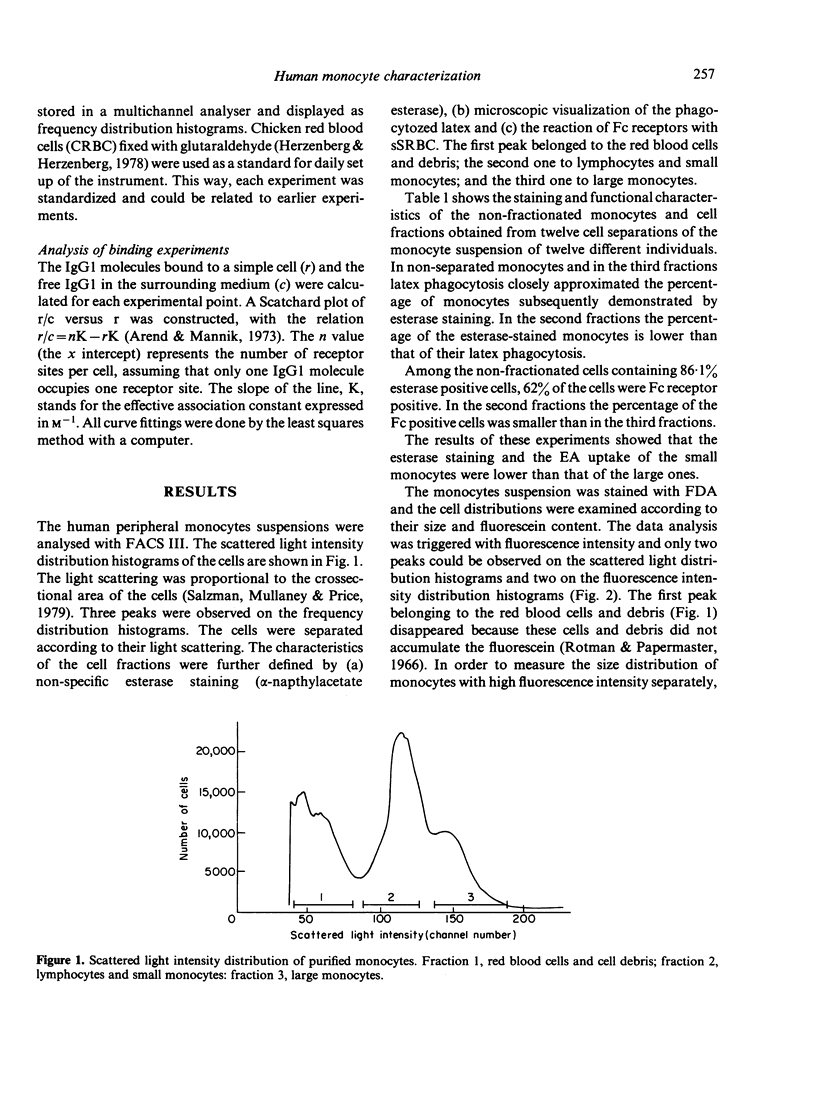
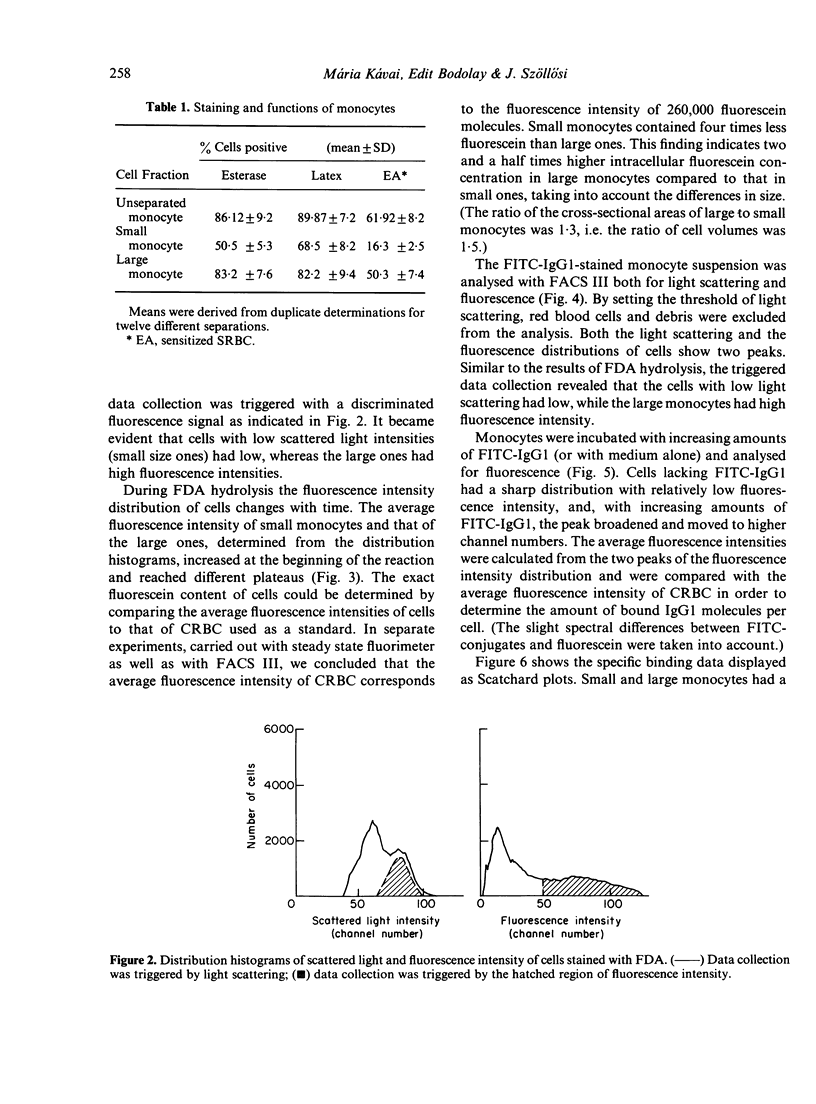
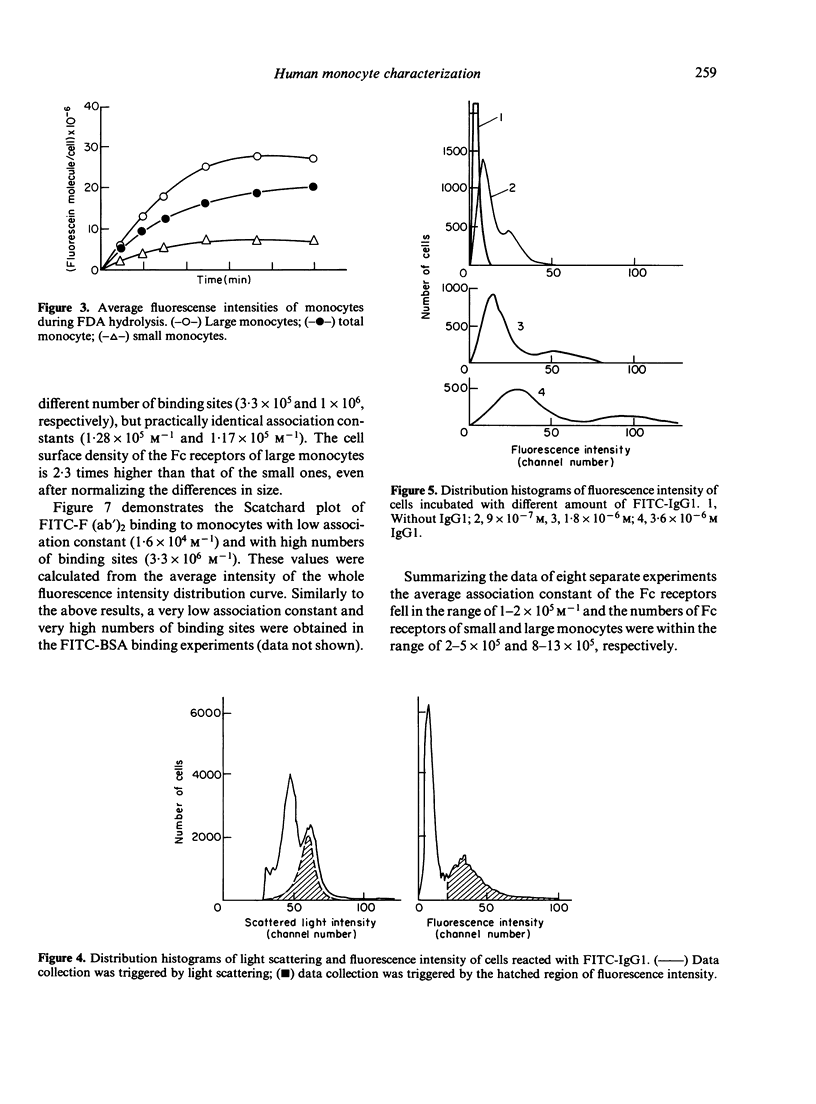
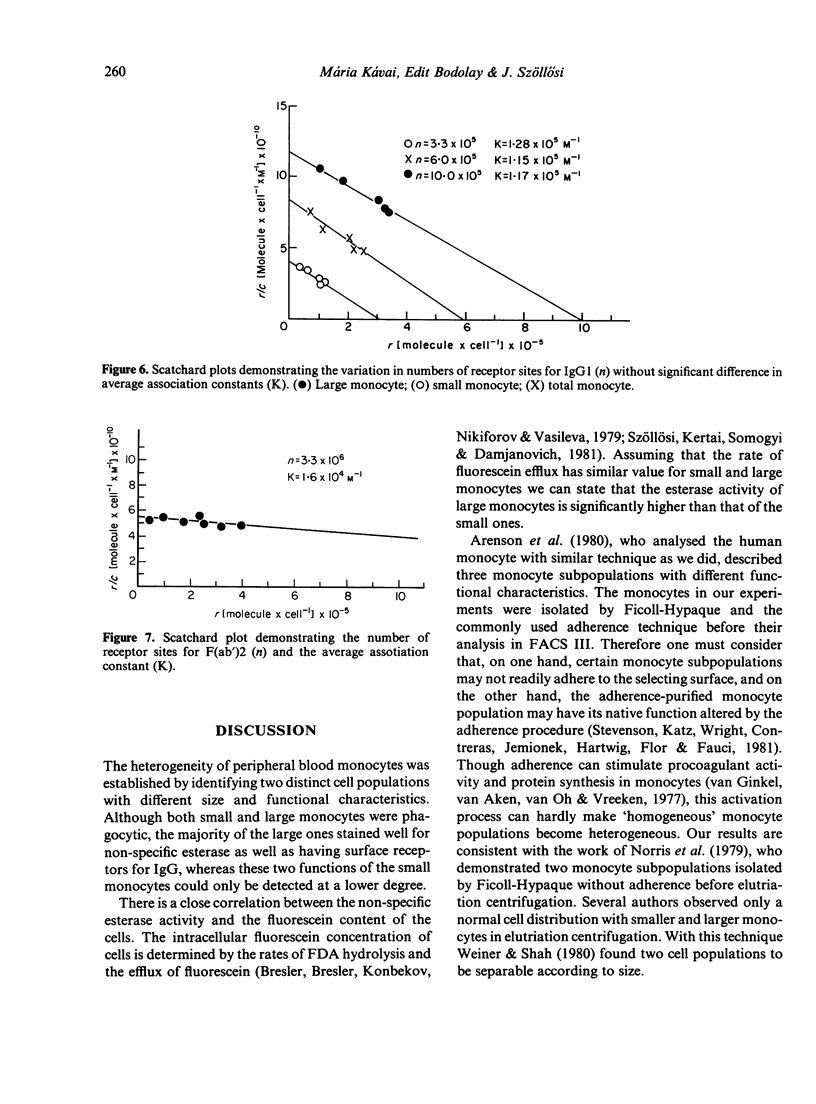
Human monocytes separated from peripheral blood by Ficoll-Hypaque and by adherence to serum-coated dishes show a bimodal volume distribution measured with a fluorescence-activated cell sorter. In the first peak of size distribution histogram of living mononuclear cells, lymphocytes and small monocytes were characterized by latex phagocytosis and non-specific esterase staining, whereas in the second peak the large monocytes dominated. The percentage of esterase stained small monocytes was lower than that of the large ones. Parallel to these data, the rate of the FDA hydrolysis of the small monocytes was lower than that of the large ones. The majority of the large monocytes reacted with sensitized sheep red blood cells (sSRBC) while only the minority of the small monocytes bound sSRBC. Scatchard plots on the binding of fluorescein isothiocyanate (FITC)-labelled human monoclonal IgG1 to the two subpopulations indicated similar association constants. K = 1 . 2 +/- 0 . 3 X 10(5) M-1. The number of Fc receptors was significantly different for the small (3 . 3 +/- 0 . 6 X 10(5)) and the large monocytes (10 +/- 1 X 10(5)).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. D., Andrews J. A., Leslie R. G., Wood N. J. The binding of human and guinea-pig IgG subclasses to homologous macrophage and monocyte Fc receptors. Immunology. 1978 Jul;35(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- An T., White W. Fc receptors on human lymphocytes detected with double-coating indirect rosette formation. Int Arch Allergy Appl Immunol. 1979;59(1):104–113. doi: 10.1159/000232246. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Arenson E. B., Jr, Epstein M. B., Seeger R. C. Volumetric and functional heterogeneity of human monocytes. J Clin Invest. 1980 Mar;65(3):613–618. doi: 10.1172/JCI109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Bresler V. M., Kazbekov E. N., Nikiforov A. A., Vasilieva N. N. On the active transport of organic acids (fluorescein) in the choroid plexus of the rabbit. Biochim Biophys Acta. 1979 Jan 5;550(1):110–119. doi: 10.1016/0005-2736(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Kwan D., Epstein M. B., Norman A. Studies on human monocytes with a multiparameter cell sorter. J Histochem Cytochem. 1976 Jan;24(1):355–362. doi: 10.1177/24.1.56390. [DOI] [PubMed] [Google Scholar]
- Kávai M., Laczkó J., Csaba B. Functional heterogeneity of macrophages. Immunology. 1979 Apr;36(4):729–732. [PMC free article] [PubMed] [Google Scholar]
- Manconi P. E., Marrosu M. G., Paghi L., Correale G., Zaccheo D. Alpha-naphthyl acetate esterase activity in human lymphocytes: distribution in lymphocyte subpopulations and in mitogen-activated cells. Scand J Immunol. 1979;9(2):99–104. doi: 10.1111/j.1365-3083.1979.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Neumann C., Sorg C. Heterogeneity of murine macrophages in response to interferon inducers. Immunobiology. 1981;158(4):320–329. doi: 10.1016/S0171-2985(81)80003-4. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Morris R. M., Sanderson R. J., Kohler P. F. Isolation of functional subsets of human peripheral blood monocytes. J Immunol. 1979 Jul;123(1):166–172. [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllösi J., Kertai P., Somogyi B., Damjanovich S. Characterization of living normal and leukemic mouse lymphocytes by fluorescein diacetate. J Histochem Cytochem. 1981 Apr;29(4):503–510. doi: 10.1177/29.4.7252124. [DOI] [PubMed] [Google Scholar]
- Tzehoval E., De Baetselier P., Feldman M., Segal S. The peritoneal antigen-presenting macrophage: control and immunogenic properties of distinct subpopulations. Eur J Immunol. 1981 Apr;11(4):323–328. doi: 10.1002/eji.1830110411. [DOI] [PubMed] [Google Scholar]
- Weston W. L., Dustin R. D., Hecht S. K. Quantitative assays of human monocyte-macrophage function. J Immunol Methods. 1975 Sep;8(3):213–222. doi: 10.1016/0022-1759(75)90114-3. [DOI] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]
- van Ginkel C. J., van Aken W. G., Oh J. I., Vreeken J. Stimulation of monocyte procoagulant activity by adherence to different surfaces. Br J Haematol. 1977 Sep;37(1):35–45. [PubMed] [Google Scholar]


