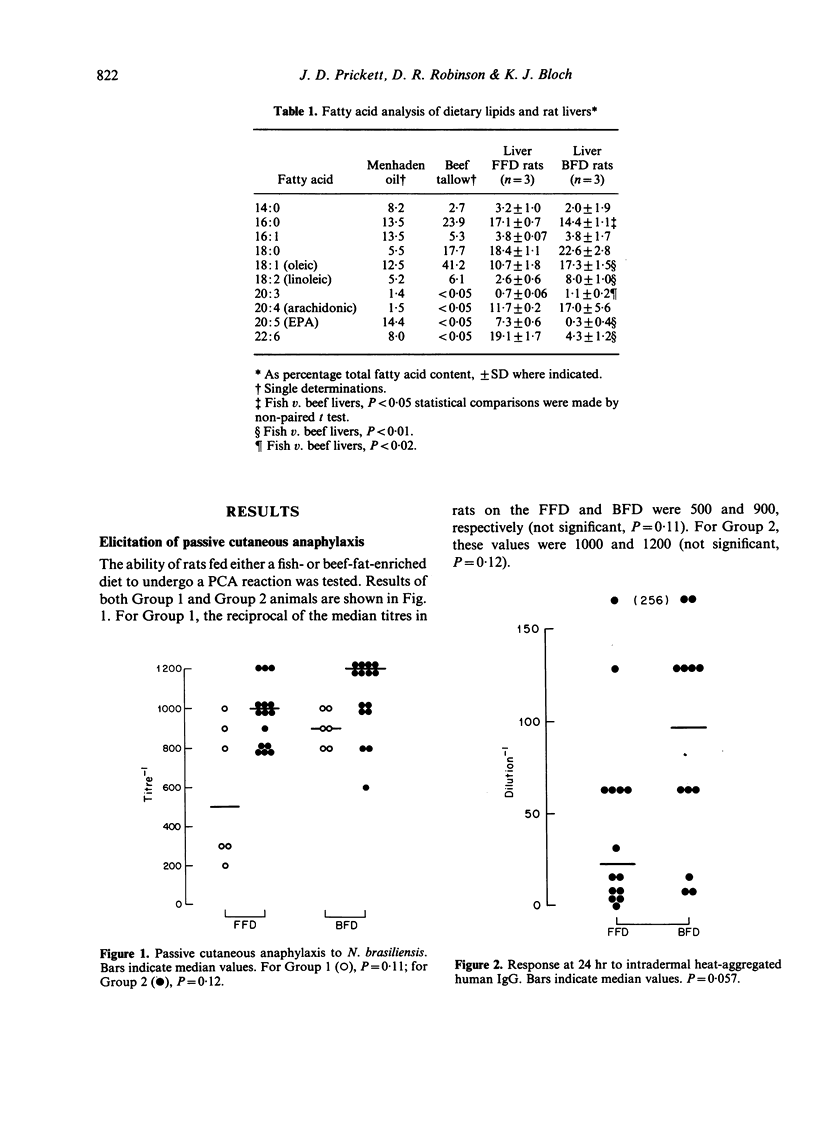
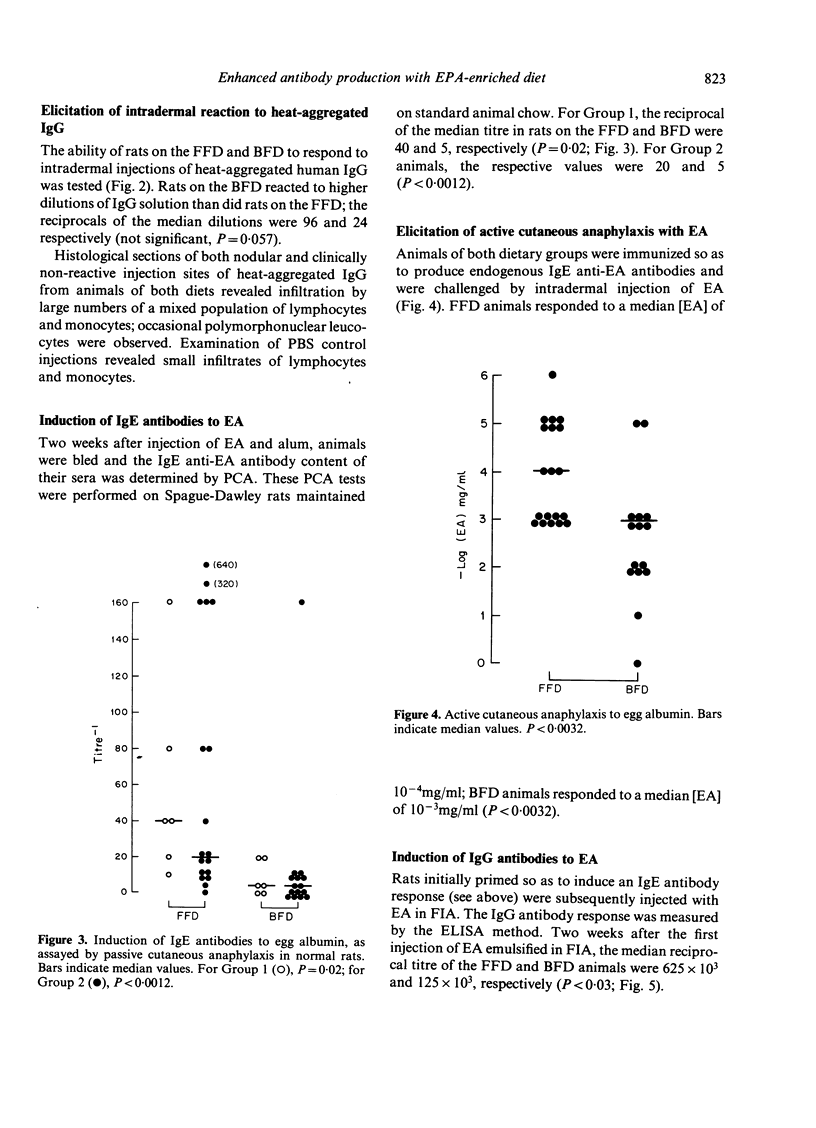
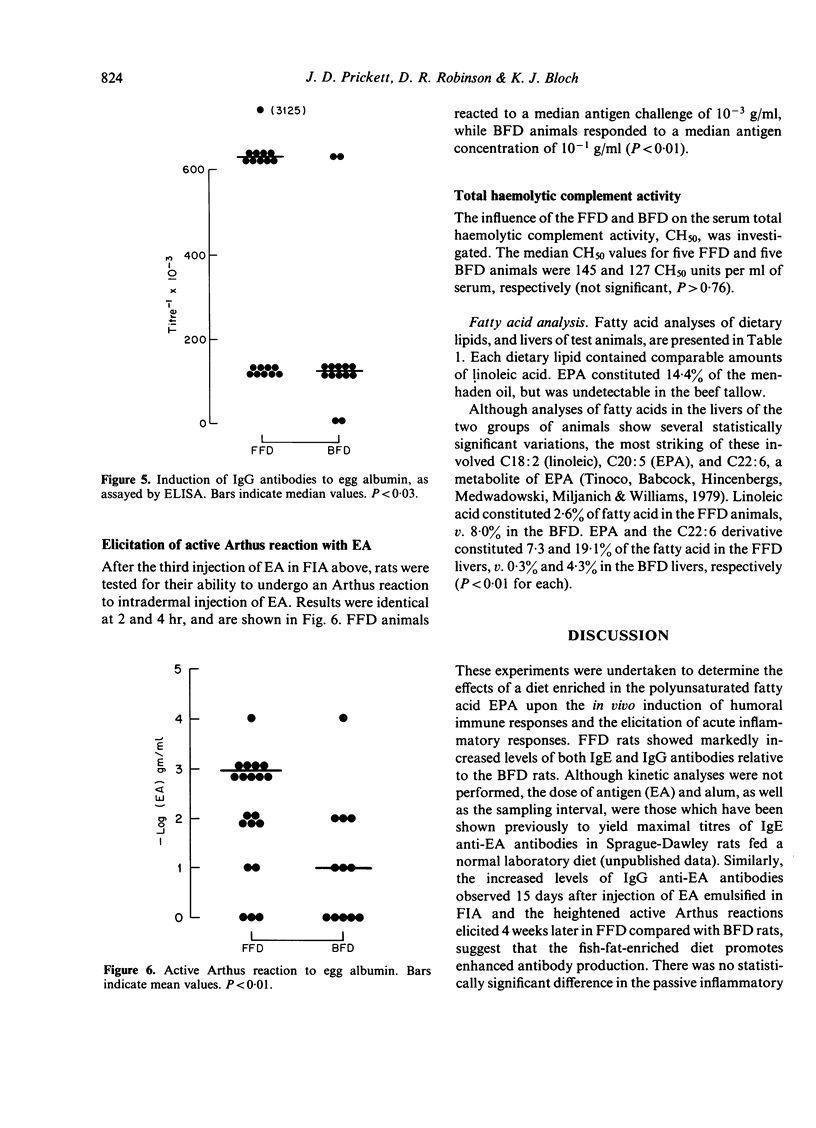
Abstract
Eicosapentaenoic acid (EPA), a polyunsaturated fatty acid analog of arachidonic acid, alters certain platelet functions controlled by prostaglandins and thromboxanes, probably by inhibiting the synthesis of these molecules from arachidonic acid. This study reports the effects of a diet enriched in EPA (fish-fat diet, FFD) as compared with a diet lacking EPA (beef-fat diet, BFD) upon certain immunological and inflammatory responses in outbred Sprague Dawley rats. Induction of antibody formation to egg albumin (EA) produced four- to eight-fold greater titres of IgE (P<0.02) and IgG (P<0.03) anti-EA antibodies in FFD rats v. BFD rats. FFD rats had heightened active cutaneous anaphylaxis to EA, responding to a median [EA] of 10-4 mg/ml, v. 10-3 mg/ml in BFD rats (P<0.0032). Similarly, active Arthus reaction to EA in FFD rats was elicited to a [EA] of 10-3 g/ml v. 10-1 g/ml in BFD rats (P<0.01). Passive inflammatory reactions, as evaluated by passive cutaneous anaphylaxis elicited with IgE antibody, and by the intradermal injection of aggregated IgG, were not significantly different between the two groups. EPA constituted 7.3% of fatty acid in the livers of FFD rats, v. 0.3% in BFD rats (P<0.01). These data demonstrate enhanced levels of IgE and IgG antibody in FFD rats, with subsequent increased active inflammatory reactivity in these animals. These alterations may be secondary to enrichment of tissue lipids with EPA, although effects due to changes in other fatty acids have not been excluded.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang H. O., Dyerberg J., Hjøorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200(1-2):69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Bloch K. J., Ohman J. L., Jr, Waltin J., Cygan R. W. Potentiated reagin response: initiation with minute doses of antigen and alum followed by infection with Nippostrongylus brasiliensis. J Immunol. 1973 Jan;110(1):197–204. [PubMed] [Google Scholar]
- Bonta I. L., Bult H., Vincent J. E., Zijlstra F. J. Acute anti-inflammatory effects of aspirin and dexamethasone in rats deprived of endogenous prostaglandin precursors. J Pharm Pharmacol. 1977 Jan;29(1):1–7. doi: 10.1111/j.2042-7158.1977.tb11227.x. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Luttenegger D. G., Dudley D. T., Buettner G. R., Spector A. A. Effect of modification of plasma membrane fatty acid composition on fluidity and methotrexate transport in L1210 murine leukemia cells. Cancer Res. 1979 May;39(5):1726–1732. [PubMed] [Google Scholar]
- CHRISTIAN C. L. Studies of aggregated gamma-globulin. II. Effect in vivo. J Immunol. 1960 Jan;84:117–121. [PubMed] [Google Scholar]
- Denko C. W. Modification of adjuvant inflammation in rats deficient in essential fatty acids. Agents Actions. 1976 Sep;6(5):636–641. doi: 10.1007/BF01971583. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Fulton A. M., Levy J. G. The possible role of prostaglandins in mediating immune suppression by nonspecific T suppressor cells. Cell Immunol. 1980 Jun;52(1):29–37. doi: 10.1016/0008-8749(80)90397-4. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Selinger D. S., Messner R. P., Reed W. P. Effect of indomethacin in vivo on humoral and cellular immunity in humans. Infect Immun. 1978 Feb;19(2):430–433. doi: 10.1128/iai.19.2.430-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN R. T. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J Nutr. 1960 Mar;70:405–410. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- HORTON E. W. ACTION OF PROSTAGLANDIN E1 ON TISSUES WHICH RESPOND TO BRADYKININ. Nature. 1963 Nov 30;200:892–893. doi: 10.1038/200892b0. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Leukotriene C5: a slow reacting substance derived from eicosapentaenoic acid. J Biol Chem. 1980 Aug 10;255(15):7093–7094. [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. P. Role of kinins and prostaglandins as mediators of functional hyperaemia. Proc R Soc Med. 1971 Jan;64(1):6–9. [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett J. D., Robinson D. R., Steinberg A. D. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981 Aug;68(2):556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Zor U., Globerson A. Function of macrophage prostaglandins in the process of phagocytosis. Adv Exp Med Biol. 1979;121(A):413–417. doi: 10.1007/978-1-4684-3593-1_37. [DOI] [PubMed] [Google Scholar]
- Siess W., Roth P., Scherer B., Kurzmann I., Böhlig B., Weber P. C. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet. 1980 Mar 1;1(8166):441–444. doi: 10.1016/s0140-6736(80)90995-2. [DOI] [PubMed] [Google Scholar]
- Tinoco J., Babcock R., Hincenbergs I., Medwadowski B., Miljanich P., Williams M. A. Linolenic acid deficiency. Lipids. 1979 Feb;14(2):166–173. doi: 10.1007/BF02533868. [DOI] [PubMed] [Google Scholar]
- Tracey D. E., Adkinson N. F., Jr Prostaglandin synthesis inhibitors potentiate the BCG-induced augmentation of natural killer cell activity. J Immunol. 1980 Jul;125(1):136–141. [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Bloch K. J. Homocytotropic antibody response in the rat infected with the nematode, Nippostrongylus brasiliensis. II. Characteristics of the immune response. J Immunol. 1968 Mar;100(3):622–628. [PubMed] [Google Scholar]
- Zimecki M., Webb D. R. The regulation of the immune response to T-independent antigens by prostaglandins and B cells. J Immunol. 1976 Dec;117(6):2158–2164. [PubMed] [Google Scholar]


