Abstract
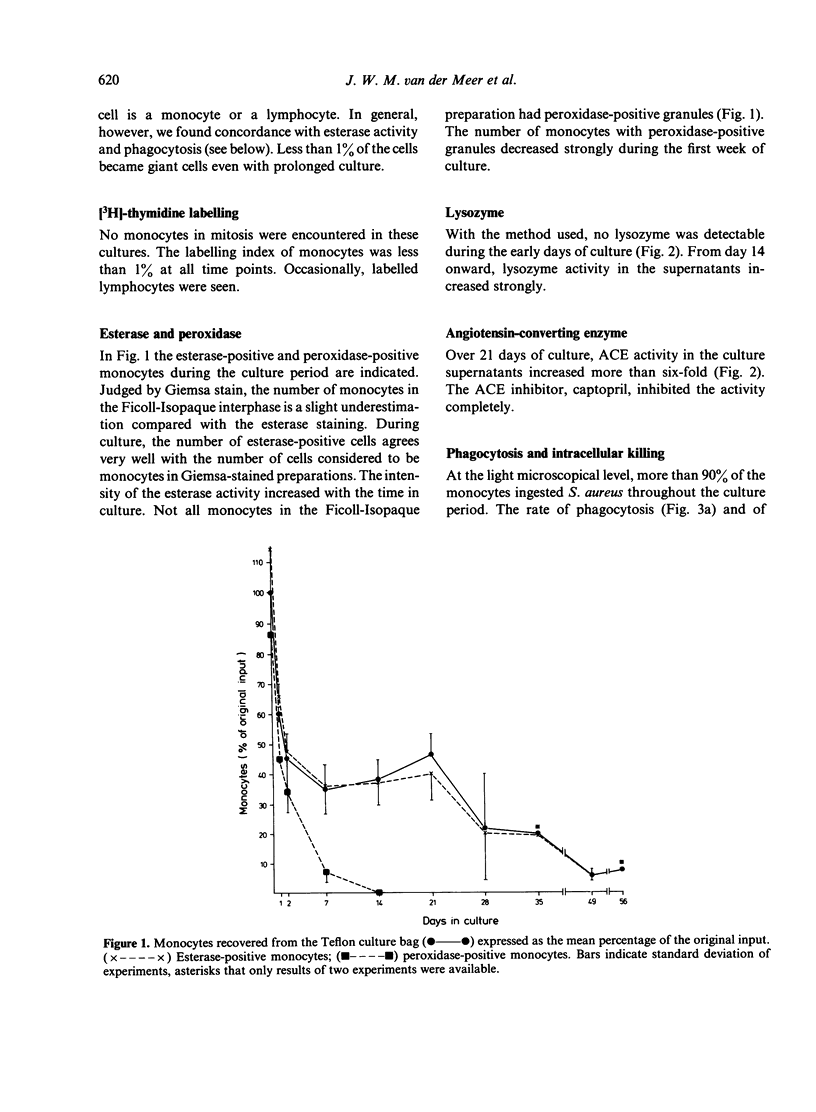
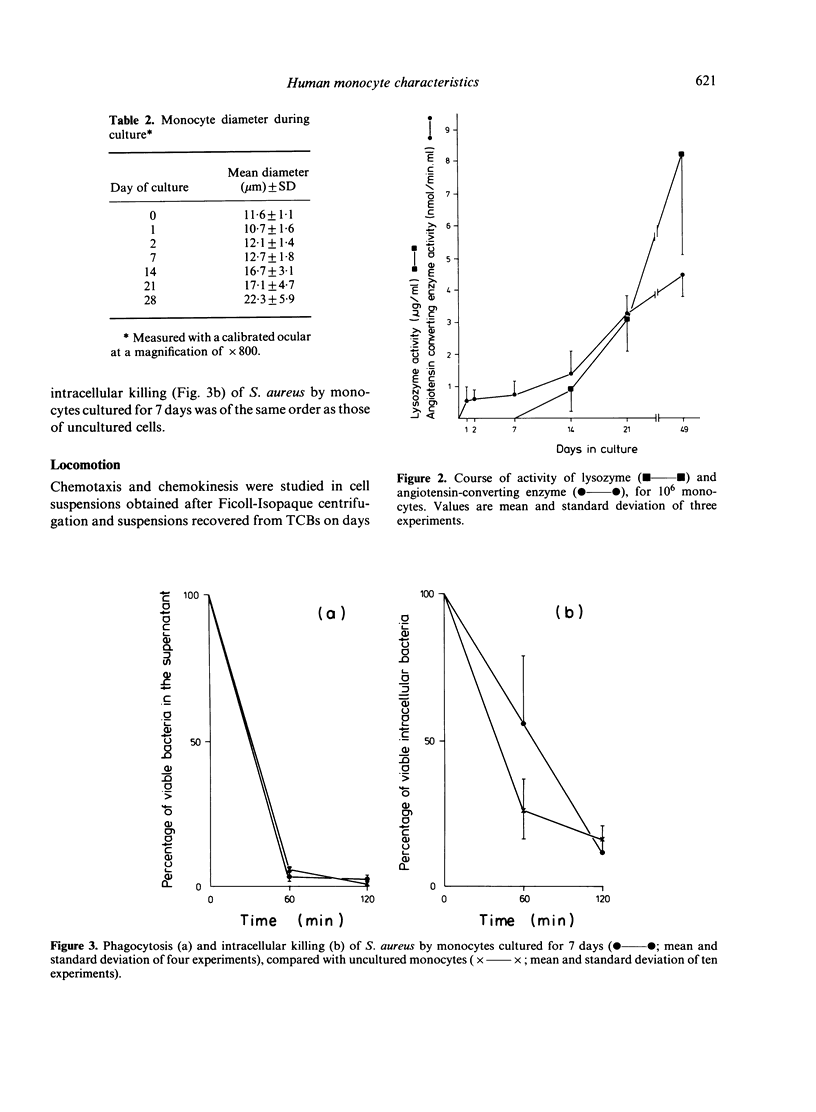
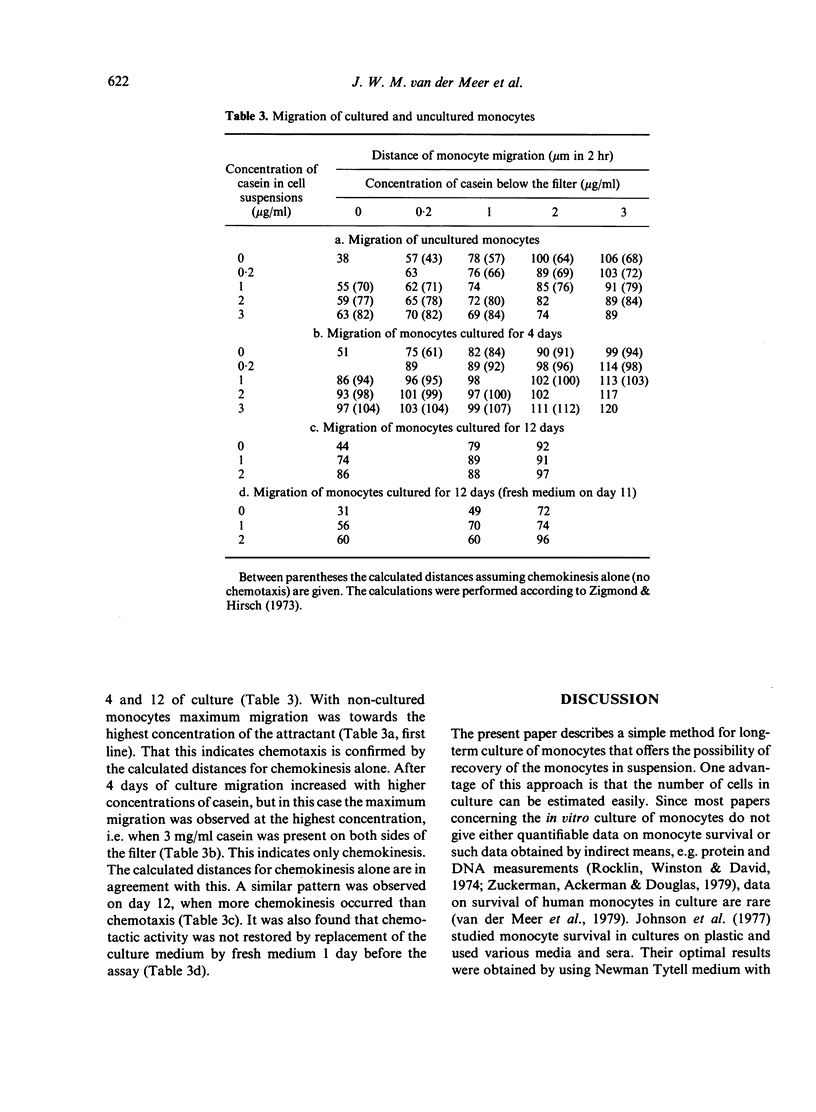
Blood monocytes from healthy volunteers were isolated by Ficoll-Isopaque centrifugation and cultured (together with lymphocytes) in medium 199 with 20% heat-inactivated newborn calf serum in a Teflon culture bag. Quantifiable data on survival showed that up to 21 days of culture, approximately 40% of the initial number of monocytes were still viable. Such cultures could be maintained for more than 8 weeks without refeeding. The monocytes exhibited the morphology of macrophages after 5-7 days of culture, and increased in size during culture. Less than 1% of the cells became giant cells even after long culture periods. Almost all cultured monocytes were positive for alpha-naphthyl butyrate esterase, whereas the peroxidase-positive granules disappeared during the first week of culture. After long culture times increasing amounts of lysozyme and angiotensin-converting enzyme were detected in the culture supernatants. Phagocytosis of staphylococci did not decrease appreciably during culture, and the same holds for intracellular killing of these bacteria. Chemotactic activity decreased during culture, whereas the chemokinetic response of the monocytes persisted.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN L., STULBERG C. S. Primary cultures of macrophages from normal human peripheral blood. Lab Invest. 1962 Dec;11:1322–1331. [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intravenous administration of heat-killed bacillus Calmette-Guérin. J Exp Med. 1981 Aug 1;154(2):235–252. doi: 10.1084/jem.154.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Bactericidal Activity of Human Macrophages: Analysis of Factors Influencing the Killing of Listeria monocytogenes. Infect Immun. 1970 Aug;2(2):156–161. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries E., Haasnoot C. J., Van der Weij J. P., Cats A. In vitro monocyte-lymphocyte interaction influenced by d-penicillamine. Clin Exp Immunol. 1982 Feb;47(2):474–480. [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J., Silverstein E. A sensitive fluorimetric assay for serum angiotensin-converting enzyme. Am J Clin Pathol. 1976 Aug;66(2):416–424. doi: 10.1093/ajcp/66.2.416. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M. N. Formation of giant cells from human monocytes cultivated on cellophane. Anat Rec. 1954 Mar;118(3):577–591. doi: 10.1002/ar.1091180307. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud T. J., Schotte C., van Furth R. Identification and characterization of the monoblast in mononuclear phagocyte colonies grown in vitro. J Exp Med. 1975 Nov 1;142(5):1180–1199. doi: 10.1084/jem.142.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. G., Malmquist J. Quantitative immunochemical determination of lysoqyme (muramidase) in serum and urine. Scand J Clin Lab Invest. 1971 May;27(3):255–261. doi: 10.3109/00365517109080216. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Loos H., Blok-Schut B., van Doorn R., Hoksbergen R., Brutel de la Rivière A., Meerhof L. A method for the recognition and separation of human blood monocytes on density gradients. Blood. 1976 Nov;48(5):731–742. [PubMed] [Google Scholar]
- Rocklin R. E., Winston C. T., David J. R. Activation of human blood monocytes by products of sensitized lymphocytes. J Clin Invest. 1974 Feb;53(2):559–564. doi: 10.1172/JCI107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel D. R., Godal T., Myrvang B., Song Y. K. Behavior of Mycobacterium leprae in human macrophages in vitro. Infect Immun. 1973 Sep;8(3):446–449. doi: 10.1128/iai.8.3.446-449.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Ackerman S. K., Douglas S. D. Long-term human peripheral blood monocyte cultures: establishment, metabolism and morphology of primary human monocyte-macrophage cell cultures. Immunology. 1979 Oct;38(2):401–411. [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., Caviles A. P., Jr, Bont W. S., Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979 Mar;122(3):1099–1107. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-Den Dulk M. M. The kinetics of promonocytes and monocytes in the bone marrow. J Exp Med. 1970 Oct 1;132(4):813–828. doi: 10.1084/jem.132.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-den Dulk M. M. Method to prove investigation of particles by macrophages with light microscopy. Scand J Immunol. 1980;12(3):265–269. doi: 10.1111/j.1365-3083.1980.tb00066.x. [DOI] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]
- van der Meer J. W., van de Gevel J. S., Elzenga-Claassen I., van Furth R. Suspension cultures of mononuclear phagocytes in the teflon culture bag. Cell Immunol. 1979 Jan;42(1):208–212. doi: 10.1016/0008-8749(79)90236-3. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


