Abstract
The effect of the injection of a synthetic platelet-activating factor (PAF-acether) upon the fate of exogenous immune aggregates was studied in normal mice. When animals received 0.5 μg of PAF-acether following the administration of 5 μg heat-aggregated human IgG (A-IgG), a 50% increase of clearance velocity was observed during the initial 5 min; thereafter, between 15 and 30 min, there was a marked increase in blood levels of immune aggregates which paralleled the increase in plasma volume observed at those times. As regards the effect of the infusion of PAF-acether on the distribution of A-IgG in the organs, hepatic uptake was not affected, kidney uptake was reduced during the first 15 min, and lung and spleen showed only minor variations. When animals were injected with 0.5 μg PAF-acether, skin trapped aggregates showed a 73% increase above control values at 5 min, followed by a fall to control values coincidently with the flood back of the aggregates into the circulation and the increase in blood volume. The injection of lower doses of PAF-acether (0.05 μg) or Diazoxide (1.5 mg) did not induce any change on the skin trapping of aggregates. In conclusion, the powerful action of PAF-acether on vascular permeability allows a reversible deposition of immune aggregates in the skin which is followed by the flood back of this material into the circulation when its pharmacological action subsides. These findings may be of some relevance to the understanding of the dynamic processes involved in the deposition of immune complexes in tissues, especially as regards skin vessels.
Full text
PDF

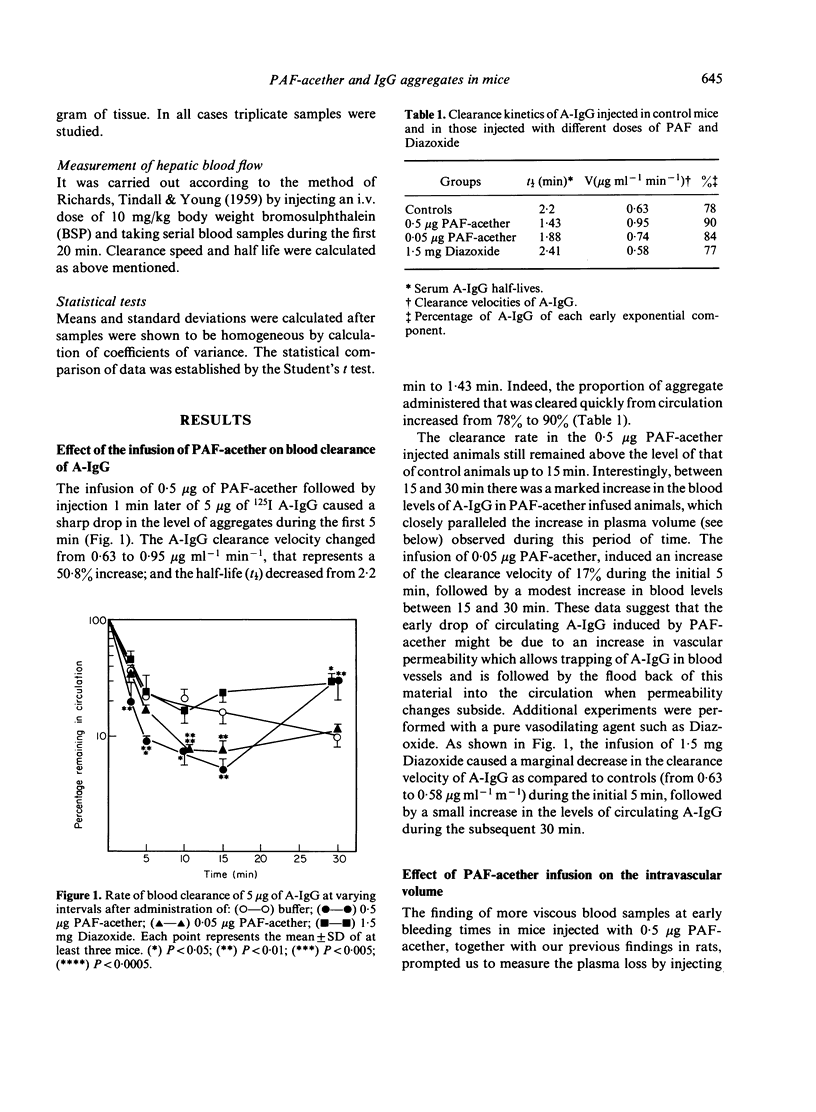
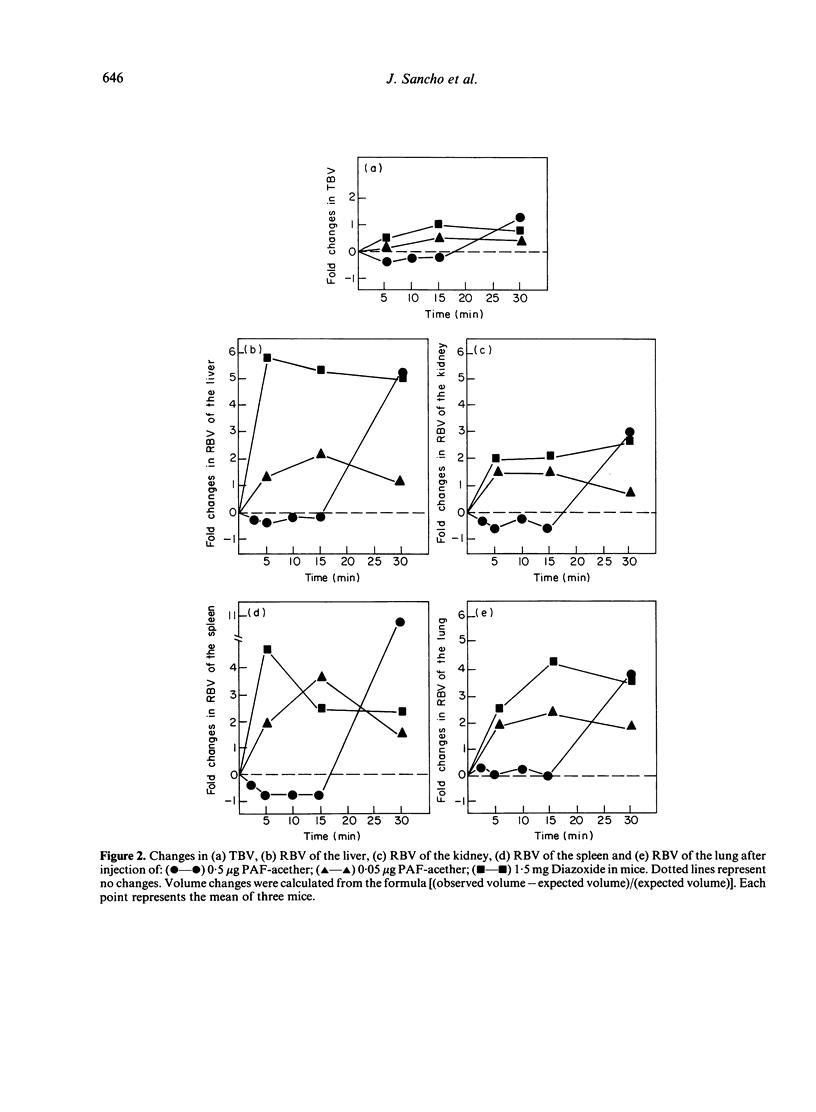
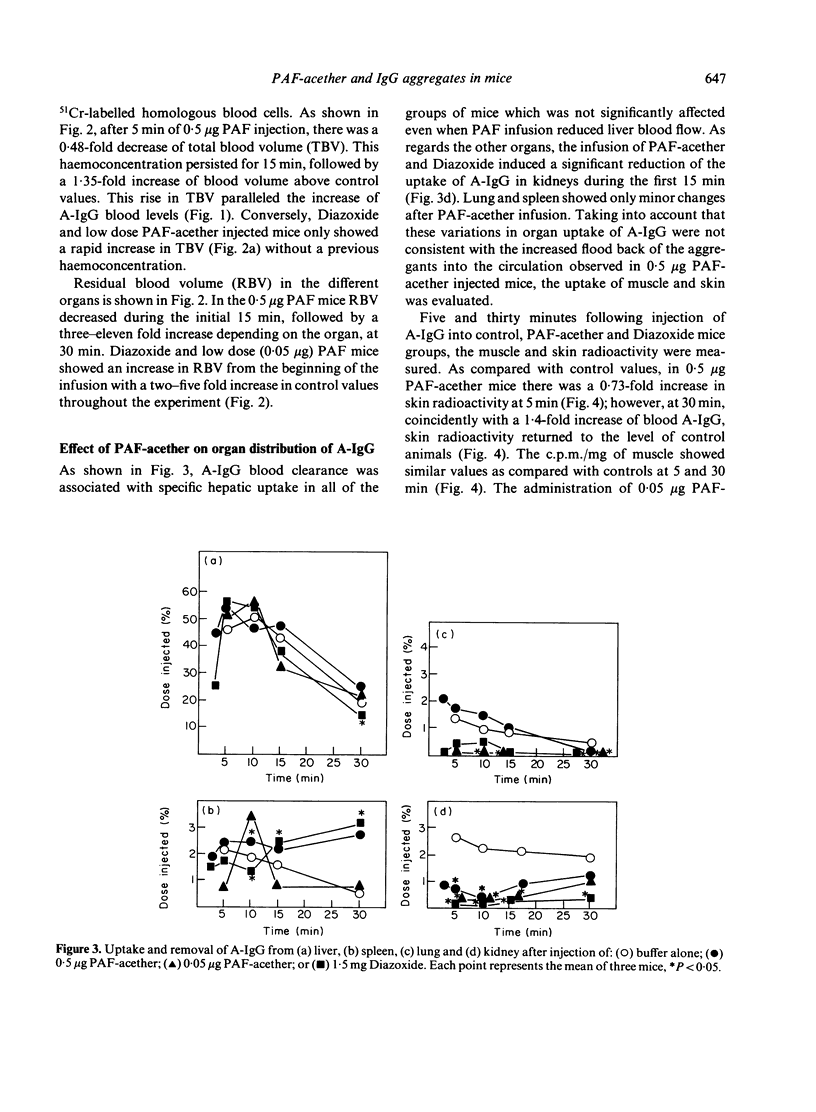
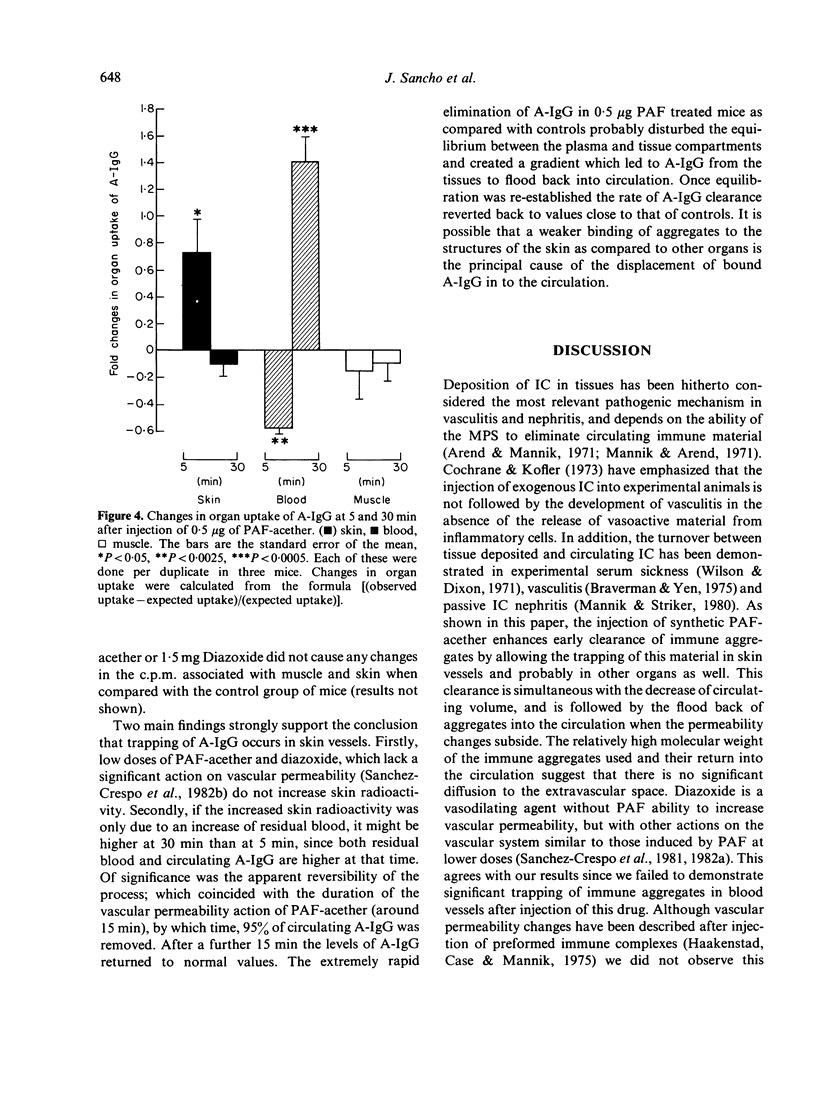


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Mannik M. Studies on antigen-antibody complexes. II. Quantification of tissue uptake of soluble complexes in normal and complement-depleted rabbits. J Immunol. 1971 Jul;107(1):63–75. [PubMed] [Google Scholar]
- Benveniste J., Egido J., Gutierrez-Millet V. Evidence for the involvement of IgE-basophil system in acute serum sickness. Clin Exp Immunol. 1976 Dec;26(3):449–456. [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Tencé M., Varenne P., Bidault J., Boullet C., Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [PubMed] [Google Scholar]
- Braverman I. M., Yen A. Demonstration of immune complexes in spontaneous and histamine-induced lesions and in normal skin of patients with leukocytoclastic angitis. J Invest Dermatol. 1975 Feb;64(2):105–112. doi: 10.1111/1523-1747.ep12510321. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Egido J., Alonso F., Sanchez Crespo M., Barat A., Hernando L. Absence of an anaphylactic vasopermeability mechanism for immune complex deposition in the Heymann nephritis of rats. Clin Exp Immunol. 1980 Oct;42(1):99–106. [PMC free article] [PubMed] [Google Scholar]
- Haakenstad A. O., Case J. B., Mannik M. Effect of cortisone on the disappearance kinetics and tissue localization of soluble immune complexes. J Immunol. 1975 Apr;114(4):1153–1160. [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A., Van Der Lelij A., Knutson W., Fleuren G. J., Vanes L. A. The influence of phagocyte function on glomerular localization of aggregated IgM in rats. Clin Exp Immunol. 1978 May;32(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- Knutson D. W., Kijlstra A., Lentz H., van Es L. A. Isolation of stable aggregates of IgG by zonal ultracentrifugation in sucrose gradients containing albumin. Immunol Commun. 1979;8(3):337–345. doi: 10.3109/08820137909050047. [DOI] [PubMed] [Google Scholar]
- Lynch J. M., Lotner G. Z., Betz S. J., Henson P. M. The release of a platelet-activating factor by stimulated rabbit neutrophils. J Immunol. 1979 Sep;123(3):1219–1226. [PubMed] [Google Scholar]
- Mannik M., Striker G. E. Removal of glomerular deposits of immune complexes in mice by administration of excess antigen. Lab Invest. 1980 May;42(5):483–489. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Benveniste J. Platelet-activating factor and macrophages. I. Evidence for the release from rat and mouse peritoneal macrophages and not from mastocytes. Eur J Immunol. 1979 May;9(5):409–415. doi: 10.1002/eji.1830090512. [DOI] [PubMed] [Google Scholar]
- Sánchez-Crespo M., Alonso F., Egido J. Platelet-activating factor in anaphylaxis and phagocytosis. I. Release from human peripheral polymorphonuclears and monocytes during the stimulation by ionophore A23187 and phagocytosis but not from degranulating basophils. Immunology. 1980 Aug;40(4):645–655. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Crespo M., Alonso F., Iñarrea P., Alvarez V., Egido J. Vascular actions of synthetic PAF-acether (a synthetic platelet-activating factor) in the rat: evidence for a platelet independent mechanism. Immunopharmacology. 1982 Apr;4(2):173–185. doi: 10.1016/0162-3109(82)90019-4. [DOI] [PubMed] [Google Scholar]


