Abstract
Small lymphocyte subsets were characterized radioautographically on the basis of several surface markers, viz. surface Ig (S-Ig), Thy-1 and Lyt (Ly-1, Ly-2 and 3) antigens in host lymphoid organs (thymus, spleen and blood) as well as at the tumour site at various stages of subcutaneous growth of two different syngeneic tumours—MPC-11 plasmacytoma and WEHI-164 fibrosarcoma in BALB/c mice. In both tumour-host combinations there was a rise in the levels of null (S-Ig-, Thy-1-) small lymphocytes as well as the Ly-23+ subset of T small lymphocytes at all the sites examined. The absolute number of these two subsets also increased excepting the case of null cell rise in the thymus which was relative. The functional potentials of Lyt subsets were explored by employing in vitro and in vivo assays. While no appreciable levels of anti-tumour cytotoxic T cells (Tc) were detectable by a 51Cr release assay in the host spleen or the tumour-draining lymph nodes at any stage of growth of MPC-11 tumour, such Tc was generated in vitro by a co-cultivation of unprimed spleen cells with irradiated MPC-11 cells. These Tc were Thy-1+ and Ly-12+, as noted from antibody+C′ mediated abrogation of cytotoxicity. These results suggested that the generation of anti-tumour Tc in vivo was suppressed in tumour-bearing hosts. The possibility of a cell-mediated suppression was tested by an adoptive transfer of thymocytes or splenocytes from tumour-bearing mice into naive or pre-immunized recipients which then received fresh tumour transplants. This procedure caused a specific enhancement of tumour growth in three tumour-host combinations: MPC-11 or WEHI-164 tumour in BALB/c mice and W-1 fibrosarcoma in CBA mice. The suppressor lineage lymphocytes appearing in vivo were found to be Thy-1+ and Ly-1-, 2+, as noted from antibody +C′ mediated abrogation of their tumour-growth promoting ability. They appeared earlier (7 days) in the thymus and later (>2 weeks) in the spleen and then persisted during the tumour lifetime. The parallel kinetics of the increase in the overall level of Ly-23+ cells and the appearance of Ly-2(3)+ suppressor lineage T cells in tumour-bearing hosts may indicate that studies of T-cell surface markers may be useful in predicting changes in the functional lymphocyte subsets.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betel I., Mathieson B. J., Sharrow S. O., Asofsky R. Distribution of Lyt phenotypes in thymocyte subpopulations as measured by flow microfluorometry: selective enrichment of Lyt 1+23- thymocytes. J Immunol. 1980 May;124(5):2209–2217. [PubMed] [Google Scholar]
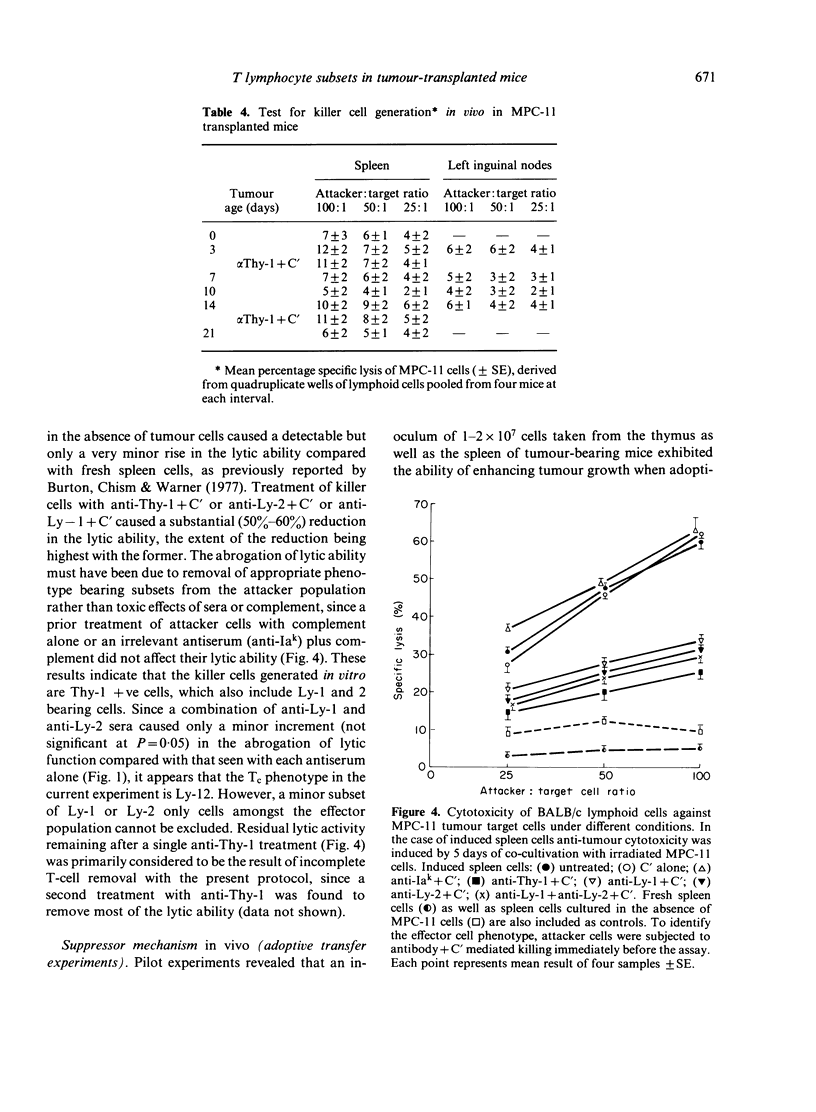
- Burton R. C., Chism S. E., Warner N. L. In vitro induction of tumor specific immunity. VII. Does autosensitization occur with in vitro culture of T lymphocytes? J Immunol. 1977 Oct;119(4):1329–1339. [PubMed] [Google Scholar]
- Burton R. C., Warner N. L. Tumor immunity to murine plasma cell tumors. III. Detection of common and unique tumor-associated antigens on BALB/c, C3H, and NZB plasmacytomas by in vivo and in vitro induction of tumor-immune responses. J Natl Cancer Inst. 1977 Mar;58(3):701–709. doi: 10.1093/jnci/58.3.701. [DOI] [PubMed] [Google Scholar]
- Burton R., Thompson J., Warner N. L. In vitro induction of tumour-specific immunity. I. Development of optimal conditions for induction and assay of cytotoxic lymphocytes. J Immunol Methods. 1975;8(1-2):133–149. doi: 10.1016/0022-1759(75)90090-3. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Development and function of subclasses of T cells. J Reticuloendothel Soc. 1975 Feb;17(2):115–118. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. Regulation of the immune response by T-cell subclasses. Contemp Top Immunobiol. 1977;7:47–67. doi: 10.1007/978-1-4684-3054-7_2. [DOI] [PubMed] [Google Scholar]
- Chatterjee-Hasrouni S., Santer V., Lala P. K. Characterization of maternal small lymphocyte subsets during allogeneic pregnancy in the mouse. Cell Immunol. 1980 Mar 15;50(2):290–304. doi: 10.1016/0008-8749(80)90284-1. [DOI] [PubMed] [Google Scholar]
- Cobleigh M. A., Braun D. P., Harris J. E. Quantitation of lymphocytes and T-cell subsets in patients with disseminated cancer. J Natl Cancer Inst. 1980 May;64(5):1041–1045. [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regualtion of the immune response to tumor antigens. I. Immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):791–799. [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regulation of the immune response to tumor antigens. II. The nature of immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):800–806. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnis S., Lala P. K. Surface markers of small lymphocytes appearing in the mouse Ehrlich ascites tumour, host spleen and blood. Immunology. 1978 Mar;34(3):487–499. [PMC free article] [PubMed] [Google Scholar]
- Giorgi J. V., Warner N. L. Continuous cytotoxic T cell lines reactive against murine plasmacytoma tumor-associated antigens. J Immunol. 1981 Jan;126(1):322–330. [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Fujimoto S., Sehon A. H. Regulation of the immune response to tumor antigens. III. Characterization of thymic suppressor factor(s) produced by tumor-bearing hosts. J Immunol. 1977 Aug;119(2):757–764. [PubMed] [Google Scholar]
- Hogarth P. M., Potter T. A., Cornell F. N., McLachlan R., McKenzie I. F. Monoclonal antibodies to murine cell surface antigens. I. Lyt-1.1. J Immunol. 1980 Oct;125(4):1618–1624. [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kaizer L., Lala P. K. Post-mitotic age of monocuclear cells migrating into TA-3(St) solid tumors. Cell Tissue Kinet. 1977 May;10(3):279–288. doi: 10.1111/j.1365-2184.1977.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Kaszubowski P. A., Husby G., Tung K. S., Williams R. C., Jr T-lymphocyte subpopulations in peripheral blood and tissues of cancer patients. Cancer Res. 1980 Dec;40(12):4648–4657. [PubMed] [Google Scholar]
- Lala P. K. Dynamics of leukocyte migration into the mouse ascites tumor. Cell Tissue Kinet. 1974 May;7(3):293–304. doi: 10.1111/j.1365-2184.1974.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Garnis S., Kaizer L., Jacobs S., Santer V. Characterization of lymphocytes invading experimental tumors. Adv Exp Med Biol. 1979;114:777–781. doi: 10.1007/978-1-4615-9101-6_127. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Johnson G. R., Battye F. L., Nossal G. J. Maturation of B lymphocytes. I. Concurrent appearance of increasing Ig, Ia, and mitogen responsiveness. J Immunol. 1979 Jan;122(1):334–341. [PubMed] [Google Scholar]
- Lala P. K., Kaizer L. Surface markers of small lymphocytes appearing in murine TA-3(St) solid tumors, host spleen, and blood. J Natl Cancer Inst. 1977 Jul;59(1):237–244. doi: 10.1093/jnci/59.1.237. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Patt H. M. Cytokinetic analysis of tumor growth. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1735–1742. doi: 10.1073/pnas.56.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala P. K., Terrin M., Lind C., Kaizer L. Hemopoietic redistribution in tumor-bearing mice. Exp Hematol. 1978 Mar;6(3):283–298. [PubMed] [Google Scholar]
- Levy J. G., Maier T., Kilburn D. G. Further characterization of thymic suppressor cells and a factor that suppress the generation of cells cytotoxic for a syngeneic tumor in DBA/2 mice. J Immunol. 1979 Mar;122(3):766–771. [PubMed] [Google Scholar]
- Maier T., Levy J. G., Kilburn D. G. The Lyt phenotype of cells involved in the cytotoxic response to syngeneic tumor and of tumor-specific suppressor cells. Cell Immunol. 1980 Dec;56(2):392–399. doi: 10.1016/0008-8749(80)90115-x. [DOI] [PubMed] [Google Scholar]
- Mathieson B. J., Sharrow S. O., Campbell P. S., Asofsky R. An Lyt differentiated thymocyte subpopulation detected by flow microfluorometry. Nature. 1979 Feb 8;277(5696):478–480. doi: 10.1038/277478a0. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Paetkau V. Generation of cytotoxic lymphocytes to syngeneic tumor by using co-stimulator (Interleukin 2). J Immunol. 1980 Nov;125(5):1897–1903. [PubMed] [Google Scholar]
- Nelson M., Nelson D. S., McKenzie I. F., Blanden R. V. Thy and Ly markers on lymphocytes initiating tumor rejection. Cell Immunol. 1981 May 1;60(1):34–42. doi: 10.1016/0008-8749(81)90245-8. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Greene M. I. T cell subset interactions in the regulation of syngeneic tumor immunity. Fed Proc. 1981 Jan;40(1):39–44. [PubMed] [Google Scholar]
- Röllinghoff M., Warner N. L. Specificity of in vivo tumor rejection assessed by mixing immune spleen cells with target and unrelated tumor cells. Proc Soc Exp Biol Med. 1973 Dec;144(3):813–818. doi: 10.3181/00379727-144-37688. [DOI] [PubMed] [Google Scholar]
- Sandrin M. S., Potter T. A., Morgan G. M., McKenzie I. F. Detection of mouse alloantibodies by rosetting with protein A-coated sheep red blood cells. Transplantation. 1978 Aug;26(2):126–130. doi: 10.1097/00007890-197808000-00013. [DOI] [PubMed] [Google Scholar]
- Santer V., Mastromarino J. H., Lala P. K. Characterization of lymphocyte subsets in spontaneous mouse mammary tumors and host lymphoid organs. Int J Cancer. 1980 Jan 15;25(1):159–168. doi: 10.1002/ijc.2910250122. [DOI] [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Bean M. A., Old L. J., Oettgen H. F. Ly phenotype of cytotoxic T cells for syngeneic tumor. J Exp Med. 1976 Oct 1;144(4):1116–1120. doi: 10.1084/jem.144.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Shen F. W. Role of different T cells sets in the rejection of syngeneic chemically induced tumors. J Immunol. 1979 Mar;122(3):1162–1165. [PubMed] [Google Scholar]
- Stutman O., Shen F. W., Boyse E. A. Ly phenotype of T cells cytotoxic for syngeneic mouse mammary tumors: evidence for T cell interactions. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5667–5671. doi: 10.1073/pnas.74.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei F., Levy J. G., Kilburn D. G. Characterization of suppressor cells in mice bearing syngeneic mastocytoma. J Immunol. 1977 Feb;118(2):412–417. [PubMed] [Google Scholar]
- Takei F., Levy J. G., Kilburn D. G. In vitro induction of cytotoxicity against syngeneic mastocytoma and its suppression by spleen and thymus cells from tumor-bearing mice. J Immunol. 1976 Feb;116(2):288–293. [PubMed] [Google Scholar]
- Treves A. J., Carnaud C., Trainin N., Feldman M., Cohen I. R. Enhancing T lymphocytes from tumor-bearing mice suppress host resistance to a syngeneic tumor. Eur J Immunol. 1974 Nov;4(11):722–727. doi: 10.1002/eji.1830041104. [DOI] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. In vitro induction of tumor-specific immunity. I. Parameters of activation and cytotoxic reactivity of mouse lymphoid cells immunized in vitro against syngeneic and allogeneic plasma cell tumors. J Exp Med. 1973 Jul 1;138(1):1–15. doi: 10.1084/jem.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. C., Berczi I., Sehon A. H. Effector and enhancing lymphoid cells in plasmacytoma-bearing mice. I. Methodological studies on the Winn assay. Int J Cancer. 1980 Apr 15;25(4):487–492. doi: 10.1002/ijc.2910250410. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Fujimoto S., Tada T. Differential activation of cytotoxic and suppressor T cells against syngeneic tumors in the mouse. J Immunol. 1979 Oct;123(4):1653–1658. [PubMed] [Google Scholar]


