Abstract
Highly aggressive, rapidly growing tumors are exposed to hypoxia or even anoxia which occurs as a consequence of inadequate blood supply. Both hypoxia and consecutive hypoxia/reoxygenation exert a variety of influences on tumor cell biology. Among these are activation of certain signal transduction pathways and gene regulatory mechanisms, induction of selection processes for gene mutations, tumor cell apoptosis and tumor angiogenesis. Most of these mechanisms contribute to tumor progression. Therefore, tissue hypoxia has been regarded as a central factor for tumor aggressiveness and metastasis. In this review, we summarize the current knowledge about the molecular mechanisms induced by tumor cell hypoxia with a special emphasis on intracellular signal transduction, gene regulation, angiogenesis and apoptosis. Interfering with these pathways might open perspectives for future innovative treatment of highly aggressive metastasizing tumors.
Keywords: Hypoxia, gene regulation, signal transduction, angiogenesis, apoptosis
Review
Introduction
Hypoxic areas are a common feature of rapidly growing malignant tumors and their metastases. Tissue hypoxia due to inadequate blood supply is supposed to occur very early during tumor development beginning at a tumor diameter of a few millimeter [1-3]. Interestingly, even after neovascularization oxygen supply generally stays behind the demands of the tumor, and thus, hypoxia remains a constant feature of these tumors [3]. Hypoxia not only accounts for tissue necrosis but has also an strong impact on tumor cell biology [4]. In particular, tissue hypoxia contributes to tumor progression in a variety of ways [for review, see [4,5]]. Thus, the hypoxic tumor cell response is of paramount importance for the understanding of tumor progression.
Up to now, in eukaryotic cells a cellular sensor for hypoxia has not been identified. However, some of the downstream effector pathways, including intracellular signal transduction cascades, which interfered with gene regulation under hypoxia, had been identified. In particular, members of the family of mitogen activated protein (MAP) kinases were shown to be involved in the transduction of the hypoxic signal [6-8]. Among the genes which were induced by hypoxia, and might even be dramatically upregulated, recent interest focused on those involved in tumor angiogenesis. However, hypoxia was not only of importance for angiogenesis-induction, but also exerted effects on cell-cell and cell extracellular matrix interaction. It had been shown that hypoxia/aglycemia downregulated E-cadherin in brain microvessel endothelial cells [9]. Since loss of cell-cell contact mediated via cadherins had been regarded as an initial step during tumor progression this phenomenon might be of relevance for hypoxic induction of tumor progression.
Tumor hypoxia also selects for gene mutations in tumor cells. In particular, mutations occurred in genes involved in the process of apoptosis. It could be shown that repeated exposure to low oxygen tension selected for p53 mutations and rendered tumor cells resistant to hypoxia-induced apoptosis [10]. It is further well documented that low oxygen tension confers resistance of tumors to irradiation therapy and may thereby contribute to tumor aggressiveness [for review, see [4]].
This review focuses on the molecular mechanisms involved in hypoxic signal transduction, gene regulation, angiogenesis factor production and apoptosis regulation in tumor cells. A better understanding of these processes might open perspectives for future tumor therapy.
Signal transduction
Extracellular stimuli that interfere with gene expression and gene regulation are mediated by different intracellular signalling cascades [for review, see [11,12]]. One of the most intensively studied signalling pathway is the mitogenic Ras/Raf/MEK/ERK cascade, which responds to growth factors and factors inducing cellular differentiation, such as epidermal growth factor (EGF) and platelet derived growth factor (PDGF) [13] (Fig. 1). Downstream targets of this cascade are well-known transcription factors such as AP-1, CREP and Elk. Parallel organized kinase cascades which particularly respond to cellular stresses, such as cellular injury by heat, UV and ionizing irradiation, and osmotic shock have been identified [for review, see [14,15]]. However, these cascades also respond to inflammatory cytokines, such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α. Members of these pathways are the stress-activated protein kinases (SAPK, also termed c-Jun N-terminal kinases, JNK) and the p38 kinase (Fig. 1).
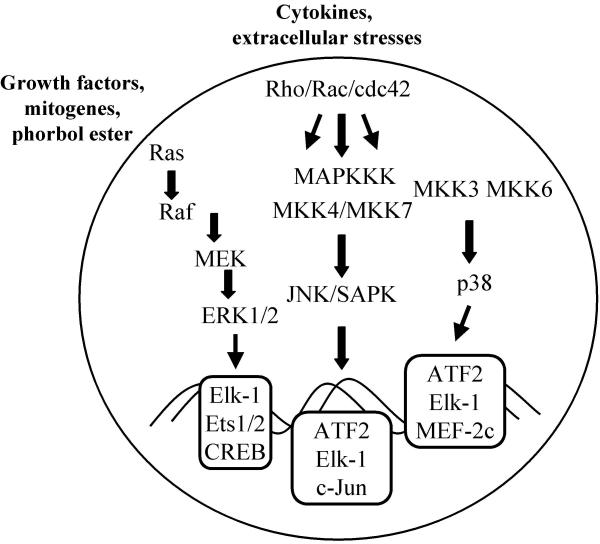
Figure 1.
MAP kinase signalling pathways. Major pathways that transfer extracellular signals to the nucleus are the MAP kinase signalling pathways. The extracellular stimuli may be heterogeneous, deriving from exposure of cells to growth factors, phorbol esters, cytokines, or cellular stresses, such as osmotic shock and γ-irradiation. In principal, the Ras-Raf-MEK-ERK pathway transduces mitogenic signals involved in cellular proliferation or differentiation. The JNK/SAPK and p38 pathways regulate the cellular inflammatory or stress response. There are interactions between both pathways on MAPK kinase kinase (MAPKKK) levels immediately upstream of MEK (not indicated in the presented scheme). The downstream targets of the MAP kinase signalling pathways are the MAP kinases, ERK, JNK/SAPK and p38, which directly or indirectly interfere with transcription factors, such as Elk-1, ATF2 or cJun for activation of gene transcription. Upstream signalling components include the family of Rho GTPases such as Rho, Rac and Cdc42 which interfere with MAPKKK. Cellular stresses such as hypoxia may activate JNK/SAPK and p38 pathways which exert influence on cJun and ATF-2 activation.
Since hypoxia is a typical stress factor for rapidly growing tumor cells, it was tempting to speculate, whether hypoxia might induce JNK/SAPK and/or p38 activation in tumors. Indeed, it could be shown that hypoxia and hypoxia/reoxygenation signalling involved members of the MAP family of signalling kinases [6-8]. Hypoxia activated the JNK/SAPK and p38 stress kinases in human squamous carcinoma cells in vitro [7]. This activation further led to a phosphorylation/activation of ATF-2 transcription factor. Moreover, in this study hypoxia-induced mitogen-activated protein kinase phosphatase (MKP)-1 mRNA expression and MKP-1 activity. MKP-1 antagonizes SAPK activity and its enhanced expression/activity under hypoxia had been regarded as one possible explanation for the rapid decline of JNK/SAPK activity after 4 hours [7].
We were able to show that stress activated protein kinase, JNK/SAPK, was activated under hypoxia in low aggressive melanoma cells [8]. Interestingly, low aggressive melanoma cell lines showed strong hypoxia-inducibility, while highly aggressive melanoma cell lines were much less responsive [8]. Thus, responsiveness of MAPK signalling pathways to hypoxia might be a tumor stage-dependent phenomenon. In further experiments, it could be shown that JNK/SAPK activation was involved in apoptosis regulation in low aggressive melanoma cells [8]. One feasible explanation for a tumor-stage dependent JNK/SAPK activation might be that late-stage tumor cells often show an independent, deregulated growth. Thus, the "classic" hypoxic signal transduction involving JNK/SAPK might be lost in these cells.
In rat cardiac myocytes hypoxia induced the redistribution of specific protein kinase C isoforms from the soluble to the particulate compartiment. These findings were suggestive for an activation of protein kinase C pathways under hypoxia [16]. In this study, inhibition of phospholipase C prevented protein kinase C redistribution. It is well accepted that protein kinase C isoforms are able to activate the Ras/Raf/MEK/ERK signalling pathway on the level of the Ras GTPase and Raf kinase. Thus, hypoxic signalling might involve protein kinase C as a possible link to MAPK signalling pathways. We were able to show that hypoxia activated p38 signalling pathways in low aggressive melanoma cells [17]. P38 activation was also observed under hypoxia/reoxygenation conditions in these cells. The physiological relevance of these findings, however, remains to be defined. However, it had been suggested that activation of p38 under hypoxia might be involved in apoptosis regulation, since it had been shown that p38 mediated apoptosis under conditions of oxidative stress [18].
One of the main downstream targets of JNK/SAPK kinase signalling is cJun, a well known member of the Jun/Fos family of transcription factors. Both, cJun and cFos may act as oncogenes and heterodimerize to form the transcriptional active complex, activator protein (AP)-1 [12,19]. Hypoxic activation of JNK/SAPK has been shown to be able to induce cJun dependent transcription [8]. The role of AP-1 in hypoxia-induced gene regulation is described in more detail in the chapter about transcription factors.
Little is known about the hypoxic induction of members of the Ras/Raf/MEK/ERK signalling pathway. Interestingly, however, BRAF knockout mice suffer from an impaired development of the vascular system [20]. Vascular malformation on the other hand has also been described in mice lacking the arylcarbon nuclear translocator (ARNT), the heterodimeric partner of hypoxia-inducible factor (HIF)-1α [21]. Thus, although largely speculative at present, hypoxic signalling might also interfere with the Raf kinase cascade at least during embryonic development. Involvement of Braf in hypoxic signalling might be of special importance for tumor growth since activating gene mutations in the BRAF gene have been demonstrated in a variety of different tumors [22].
The role of further upstream signalling molecules of MAP kinases, such as members of the family of Rho and Ras GTPases, has been described in oncogene signalling in various tumors [23]. Although, Ras mutations have been shown to play an outstanding role in the pathogenesis of epithelial tumors these mutations have not been identified in significant percentages in other tumors [24]. A direct activation of Ras by hypoxia has not been shown up to now. However, it could be shown in in vitro luciferase experiments that cells plated at high cell density (which induces pericellular hypoxia) activated the hypoxia response element [25]. In transfection studies, this activation could be inhibited by a dominant negative interfering Ras mutant and nitric oxide inhibitors. Moreover, the action of nitric oxide could be placed upstream of the Ras signalling pathway [25]. These findings suggest an indirect activation of Ras in hypoxic signalling via nitric oxide. In accordance with these findings, it had been shown that ERK – a further downstream effector molecule of Ras signalling-phosphorylated hypoxia inducible factor (HIF)-1α and thus contributed to enhanced VEGF expression under hypoxic conditions [26].
Another possible upstream activator of MAPK signalling pathways, the Rho GTPase RhoC, has been identified as a molecule that might confer high aggressiveness on malignant melanoma [27]. By use of DNA chip technology, RhoC was identified as one of the most interesting target genes which showed enhanced expression in highly aggressive, metastasizing melanoma cells compared with their low aggressive, non-metastasizing counterparts. The functional relevance of these findings was further demonstrated in a mouse metastasis model and in matrigel assays using RhoC transfected melanoma cells. However, the question whether hypoxia influences Rho GTPases activity in tumors has not been addressed up to now.
Gene regulation
It is well established that hypoxia induces gene expression and the number of hypoxia-inducible genes is increasing steadily. In response to hypoxia, eukaryotic cells show expression of genes which are involved in cellular metabolism (e.g. anaerobic glycolysis), erythropoiesis, cellular proliferation and survival, and vascular biology [for review, see [28]]. One major discovery in recent years was the identification and characterization of the transcription factor, hypoxia-inducible factor (HIF)-1α [29]. HIF-1α dependent gene activation is of central importance for a series of hypoxia-inducible genes, e.g. erythropoietin, lactate dehydrogenase A and vascular endothelial growth factor (VEGF) [for review, see [28,30,32]]. After the initial description of HIF-1α the molecular mechanisms that interfere with HIF-1α gene expression, protein stability, and DNA binding have attracted great interest, as interference with these mechanisms might open perspectives for new treatment strategies for rapidly growing malignant tumors (Fig. 2).
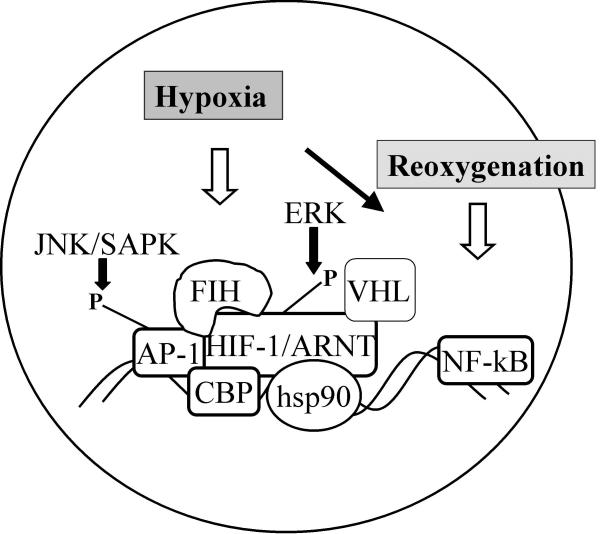
Figure 2.
Hypoxic activation of transcription factors HIF-1α, AP-1 and NF-κB. Cells exposed to hypoxia activate the cellular transcription factors HIF-1α, AP-1 and NF-κB. Under normoxic conditions the von Hippel Lindau tumor suppressor protein mediates ubiquitination and degradation of HIF-1α. This mechanism is inhibited under hypoxia. As a consequence, the HIF-1α protein is stabilized and shows enhanced expression. There are a series of factors that may interfere with HIF-1α under hypoxia, such as cJun/AP-1, heat shock protein (hsp) 90, and the transcriptional co-activator CBP/p300. Further mechanisms of HIF regulation include phosphorylation by extracellular signal regulated kinase (ERK), and phosphorylation of its interaction partner, cJun/AP-1 via stress activated protein kinase, JNK/SAPK. Recently, a factor inhibiting HIF-1α activation, FIH, has been described, representing a further level of HIF regulation. Upon reoxygenation, NF-κB, a well-known transcription factor involved in transcriptional regulation of immune response genes, is activated. However, evidence has been provided that NF-κB activation may also be induced by hypoxia.
HIF-1α heterodimerizes with HIF-1β (also termed ARNT) to form the transcriptionally active protein complex. The family of HIFs comprises two further members, HIF-2α and HIF-3α. The role of the latter for hypoxic gene regulation has not been defined so far. Further molecules that interfere with the HIF/ARNT complex are well known transcriptional coactivators, such as CBP/p300 [for review, see [31]]. HIF-1α protein is rapidly degradated and ubiquitinated under normoxic conditions, however, stabilized under hypoxic conditions. Ubiquitination is mediated by the von Hippel Lindau (VHL) tumor suppressor gene [for review, see [28]]. Accordingly, patients suffering from von Hippel Lindau syndrome – a disease characterized by a loss of the VHL gene-showed enhanced expression of HIF-1α and HIF-dependent genes, such as angiogenesis factors [32]. A typical clinical feature of these patients are vascular tumors.
More recent data showed that proline hydroxylation of HIF in the oxygen-dependent degradation domain (ODD) – which overlaps with the N-terminal transactivation domain – is of central importance for the recognition of HIF-1α by the VHL protein and subsequent ubiquitination [33]. In this study, proline hydoxylation was dependent on cellular oxygen levels and required 2-oxoglutarate and iron as co-factors.
In another recent study, a further level of HIF regulation has been described. It is known that hypoxia promotes the interaction of transcriptional coactivators such as CBP/p300 with its C-terminal transactivation domain. This interaction was shown to be inhibited by the action of a factor called FIH-1 ("factor inhibiting HIF-1") [34]. Within the C-terminal transactivation domain a conserved asparagine residue has been identified and it could be shown that this residue is hydroxylated under normoxic conditions leading to a blockage of interaction with transcriptional co-activators. It could be further shown that FIH is indeed an asparaginyl hydroxylase [35]. Interestingly, however, overexpression of FIH under hypoxia was still able to suppress HIF activity, suggesting that further mechanisms, additional to low O2 levels, might be required for FIH inactivition under hypoxia.
Posttranslational stabilization under hypoxia and enzymatic modification on prolyl and asparaginyl residues were, however, not sufficient for the regulation of the transcriptional activity of HIF-1α. Protein phosphorylation apparently plays a further important role [36]. In hamster fibroblasts it could be demonstrated that under low oxygen tension HIF-1α was directly phosphorylated and activated by the MAP kinase ERK. This phosphorylation enhanced HIF-1 dependent transcriptional activition of the VEGF gene [26,36].
Based on the current knowledge about its role in hypoxia-induced gene expression and the mechanisms of molecular regulation one could speculate that HIF-1α might be an interesting target for future tumor therapy. Indeed, the relevance of HIF-1α for tumor progression could be demonstrated [37]. In the latter paper HIF-1α wildtype H-ras-transformed mouse embryonic fibroblasts showed enhanced tumor growth in vivo compared with their HIF-1α deficient counterparts. The underlying mechanisms for the observed differences remain elusive. Interestingly, there were no differences in vascularity of tumors derived from both wildtype and HIF deficient cells. Ravi and coworkers demonstrated that human colon cancer cells deficient in p53 display enhanced tumor growth and neovascularization of tumor xenografts in nude mice [38]. It could be further shown that p53 expression exerted a negative influence on HIF-1 expression via induction of Mdm2 mediated ubiquitination and proteasomal degradation of HIF-1. In contrast, loss of p53 enhanced HIF-1 activity and VEGF expression under hypoxia. Thus, both enhanced growth and vascularity could attributed to HIF-1 expression and function. Furthermore, in another mouse tumor model, inhibition of HIF-1α activity by anti-sense technique reduced aggressive tumor growth [39]. Based on these findings, the idea was emphasized that interfering with the ubiquitination pathway and hydroxylation events might be a promissing approach for future therapeutic strategies [for review, see [40]].
Activator protein (AP)-1 is a well-known oncogene and transcription factor. AP-1 has been shown to be involved in a variety of processes linked to malignant transformation of mammalian cells [for review, see [19,41]]. However, evidence has also been provided that it may be involved in gene regulation under cellular stress, in particular under hypoxia [42] (Fig. 2). While the transcription factor NF-κB is known to play a central role in gene regulation under hypoxia/reoxygenation, AP-1 has been shown to be strongly activated under hypoxia [42]. It could be shown in a series of different tumor cells, e.g. colon cancer, glioblastoma and malignant melanoma cells that AP-1 activity may be induced by hypoxia [43-45]. AP-1 is a heterodimeric complex which may be composed of a variety of different components, among which members of the family of Jun and Fos proteins are of central importance [for review, see [19,41]]. Since cJun is targeted by the JNK/SAPK signalling this pathway may be one signalling cascade by which AP-1 activity is mediated under hypoxia. Indeed, JNK/SAPK signalling leads to AP-1 activation under hypoxia [8,46]. NF-κB activation may also be induced under hypoxia, although strongest activation was observed under hypoxia/reoxygenation [42,47]. The latter mechanism has been proposed to be independent of the degradation of the common upstream inhibitor of NF-κB, IκBα. Tyrosine hydroxylation of IκBα-which does not impact on its degradation – has been proposed as an activation mechanism under hypoxia/reoxygenation [48].
Recently, it could be demonstrated that both AP-1 and HIF cooperated in hypoxia-induced transcription [49-51]. In one study [50], using wildtype and HIF-1α nullizygous mouse embryonic fibroblasts it was shown that under chronic hypoxia c-Jun mRNA expression and phosphorylation of cJun in the N-terminal region were both dependent on the presence of HIF-1α [50]. In contrast, early and rapid induction of cJun was not dependent on HIF-1α. The underlying mechanisms remain to be investigated. However, positive feedback of hypoxia-induced cJun expression on its own transcriptional induction was excluded since the phosphorylation/activation status of cJun did not impact on the levels of cJun mRNA expression. It was suggested that genes induced via HIF-1α dependent transcription might contribute to enhanced cJun expression.
In another study, a direct cooperation of both transcription factors, cJun/AP-1 and HIF-1, has been demonstrated [51]. The investigators used in vitro luciferase assays to show that overexpression of cJun induced HIF-dependent promoter activity. This promoter activity could partially be inhibited by co-expression of TAM67, a dominant negative form of cJun. Moreover, in further immunoprecipitation studies, an association between cJun and HIF-1α could be demonstrated. In addition, evidence was provided that this interaction requires further interaction partners, since HIF-1α /cJun interaction could only be demonstrated in vivo but not in vitro, using both partners HIF-1α and cJun as in vitro translated proteins.
Direct or indirect interaction of both transcription factors cJun/AP-1 and HIF-1α might be a general mechanism for the regulation at least for some of the known hypoxia-inducible genes. In line with this, in immunoprecipitation studies we were able to co-immunoprecipitate HIF-1α with antibodies against cJun and vice versa, indicating that HIF-1α binds to cJun/AP-1 [Kunz et al., submitted]. One mechanism by which both HIF and cJun/AP-1 may interact in vivo could involve the transcriptional co-activator CBP/p300, a molecules which had been shown to bind to both molecules. Indeed, CBP/p300 recruitment to HIF has been shown to be redox-regulated [for review, see [31]].
SP-1 had been regarded as a transcription factor involved in baseline gene transcription of a variety of genes [52]. Little is known about the role of SP-1 in hypoxia-induced gene regulation. However, SP-1 has been recently shown to be involved in the transcriptional regulation of cyclooxygenase-2 in vascular endothelial cells under hypoxic conditions [53]. These studies were undertaken based on observations that cyclooxygenase-2 showed enhanced expression in vivo in hypoxic heart and vessel tissues. Overexpression of SP-1, but not SP-3, was able to induce the cyclooxygenase-2 promoter in in vitro luciferase assays. In contrast, SP-3 acted as a transcriptional repressor under hypoxia in these studies.
Taken together, the picture of the molecular mechanisms underlying hypoxia-induced gene regulation is becoming more and more complete. Hypoxic gene regulation involves transcription factors HIF-1α, AP-1, NF-κB and SP-1. Some of these probably interact directly.
Angiogenesis factor production
It is well established that local growth and metastasis of a large variety of malignant tumors are dependent on neovascularization [for review, see [1,2,54,55]]. Tumor angiogenesis requires the production and secretion of so-called angiogenesis factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet derived growth factor (PDGF) and interleukin-8 [for review, see [55,58]]. The mechanisms underlying angiogenesis factor production are, however, poorly understood. Evidence has been provided that a constitutive high expression of angiogenesis factors in isolated tumor cells or cell clones may be the first step in a selection process towards angiogenesis factor producing tumors [59]. A constitutive high expression of angiogenesis factors in tumor cells may derive from the so-called "angiogenic switch" which is supposed to happen very early during tumor development [for review, see [54]]. In accordance with this, it has been shown that a constitutive high expression of IL-8 promotes tumor growth of melanoma cells in vivo [60].
However, it has been shown for a large series of angiogenesis factors that these were strongly induced by tissue hypoxia. Based on currently available data, hypoxia inducible angiogenesis factors are VEGF, IL-8, angiogenin, FGF and PDGF [44,45,61-66]. In a recent attempt using gene expression libraries of hypoxic glioblastoma cells a series of new, as yet unknown, hypoxia-inducible genes were identified [67]. Among the genes identified that may impact on tumor angiogenesis, a gene termed "angiopoietin-related gene" was described. Its role for tumor angiogenesis awaits further investigation.
That hypoxia may indeed act as a strong inducer of angiogenesis factors in tumor cells has been shown for a variety of malignant tumors in vitro and in vivo, e.g. glioblastoma, ovarian carcinoma, malignant melanoma and squamous cell carcinoma [45,61,65,68,69]. These findings may have a profound influence on future tumor therapies and therefore have attracted great interest in recent years. A large series of clinical trials are currently underway testing various antiangiogenic compounds for their efficacy in tumor therapy [for review, see [55]].
At present, vascular endothelial growth factor (VEGF) is the best characterized angiogenesis factor. VEGF was initially described as "vascular permeability factor" [70]. Investigations on VEGF unveiled a series of different receptors with structural similarities: Flt 1 (VEGFR-1), KDR/Flk-1 (VEGFR-2), Flt 4, Tie-1 and Tek/Tie-2. The functional importance of VEGF and VEGF receptors for embryonic vasculogenesis and angiogenesis had been demonstrated in a variety of experimental models including VEGF and VEGF receptor knockout mice [71]. VEGF expression had been shown to play a particular role in the growth of glioblastomas, which show extensive vascularization [72]. A characteristic feature of VEGF expression is its inducibility by hypoxia. HIF-1α could be identified as major transcription factor involved in the hypoxic gene induction of VEGF [62]. Recently, the role of VEGFR-2 (also termed KDR/Flk-1) for the invasive growth of skin squamous cell carcinoma cells could be demonstrated [69]. In malignant keratinocyte transplants onto nude mice the invasive phenotype of these cells could be reverted by addition of blocking antibodies to VEGFR-2. Furthermore, it could be shown that VEGF overexpression in human melanoma cell lines led to increased growth of tumors subcutaneously injected into nude mice. The latter tumors were well vascularized and displayed little necrosis, while non-transfected control tumor cells formed minimally vascularized tumors with extensive necrosis [73]. In clinical trials specific peptides directed against VEGF are currently being used for antioangiogenic tumor treatment [for review, see [55]].
Angiogenin, first described in adenocarcinoma cell-conditioned medium, is a potent angiogenic factor in vivo [74]. Angiogenin is a member of the RNase superfamily targeting ribosomal and transfer RNA. Its ribonucleolytic activity is low, however, significantly contributes to its angiogenic activity. A 170 kDa cell surface angiogenin receptor has been identified mediating angiogenin induced endothelial cell proliferation [75]. In inhibition experiments with a monoclonal antibody directed against angiogenin suppression of tumor growth of human colon adenocarcinomas, lung carcinomas, and fibrosarcomas in nude mice was observed [76]. Angiogenin has also been identified as an important angiogenesis factor for melanoma progression [64]. In melanoma metastases angiogenin has been shown to be predominantly localized around necrotic/hypoxic tumor areas. In accordance with this, its induction by hypoxia could be shown in melanoma cells in vitro [64].
Interleukin-8 (IL-8) is a member of the well-known superfamily of CXC chemokines [for review, see [77]]. Originally, it was termed neutrophil-activating peptide. However, it also acts as a strong activator of lymphocytes and monocytes. IL-8 is also a well defined angiogenesis factor [78]. It could be shown that IL-8 induces neovascularization in the rabbit cornea assay. Further detailed analyses demonstrated that an aminoterminal ELR motif is central for IL-8 receptor binding and angiogenesis induction. IL-8 has been opposed to other members of the CXC chemokine family which lack the ELR motif, such as Mig-1 and IP-10. Both chemokines have no angiogenic capacity, but exert antiangiogenic effects. The molecular regulation of IL-8, especially after cytokine stimulation of target cells, has been extensively studied in the past years [79]. More recent investigations showed that IL-8 production is inducible in tumor cells by hypoxia [65,68]. We were able to demonstrate that IL-8 was strongly expressed in vivo in hypoxic melanoma metastases [45]. The molecular mechanisms underlying hypoxic gene regulation of IL-8 involved the transcription factors NF-κB and AP-1 [44,45]. IL-8 induction by hypoxia might be a general mechanism in a variety of tumors. Based on currently available data IL-8 activation by hypoxia occurred in malignant melanoma, glioblastoma and ovarian carcinoma [45,65,68].
Cyr61 is a recently identified angiogenesis factors, which was initially described as a growth factor inducible gene in mouse fibroblasts [80]. It belongs to the CCN family of immediate early genes. The acronym CCN stands for the currently best characterized family members CCN1 (CYR61), CCN2 (CTGF, connective tissue growth factor), and CCN3 (NOV, nephroblastoma overexpressed) [for review, see [81]81]. CCN family members are involved in cellular proliferation and cell adhesion. Recent investigations showed that Cyr61 acted as an angiogenesis factor [82]. In this study, it could be shown that Cyr61 stimulated endothelial cell migration in vitro and induced neovascularization of rat corneas. Moreover, in a nude mouse tumor model, transfected Cyr61 overexpressing RF-1 human gastric carcinoma cells resulted in larger and more vascularized tumors compared with their non-transfected normal counterparts.
The receptors mediating the diverse functions of Cyr61 have been investigated in more detail and it could be shown that Cyr61 binds to members of the family of integrins, e.g. αvβ3, αvβ5. Integrin receptor binding was of importance for endothelial cell activation and proliferation, however, might also account for the effects of Cyr61 on tumor cells. Very recently, it could be shown that high Cyr61 expression was associated with the aggressiveness of breast carcinoma cells lines [83]. Additionally, we were able to show that Cyr61 is hypoxia-inducible in melanoma cells and both transcription factors, AP-1 and HIF-1α, may contribute to the hypoxic induction of Cyr61 [Kunz et al., submitted]. Interestingly, also CTGF, another member of the CCN family, could be induced by hypoxia [84].
Apoptosis regulation
In malignant tumors the rate of apoptosis is high in undervascularized areas [85,86]. One feasible explanation for these findings might be that low oxygen pressure/hypoxia in the tumor microenvironment might directly induce apoptosis in tumor cells. Indeed, it could be shown in vitro that besides it various metabolic effects, hypoxia induced apoptosis in tumor cells [for review, see [87]]. The mechanisms of apoptosis regulation under hypoxia are, however, poorly understood. Evidence has been provided that the so-called mitochondrial permeability transition (MPT), presenting as a hyperpermeability of the inner mitochondrial membrane, is a central mechanism in hypoxia mediated apoptosis (Fig. 3). As a consequence of MPT induction cytochrome C is released into the cytoplasm. Cytochrome C in turn interacted with Apaf-1, a central kinase in apoptosis signalling [88]. Apaf-1 is a well known activator of downstream effector caspases, which are the main executors of apoptosis, such as caspase 9. This process could be counteracted by members of the Bcl-2 family of anti-apoptotic molecules, such as Bcl-2 itself and Bcl-xL. However, evidence had also been provided that Bax, another member of the Bcl-2 family, enhanced hypoxia-induced apoptosis. Bax was able to generate membrane pores in the outer mitochondrial membrane and thereby contribute to cytochrome C induced apoptosis [89]. The mechanisms by which members of the Bcl-2 family exert anti-apoptotic effects are not fully defined. It had been suggested that reactive oxygen metabolites/species (ROS) are the main contributors to stress-induced apoptosis and are affected by the expression of members of the Bcl-2 family. However, it could be demonstrated that Bcl-2 and Bcl-xL may protect PC12 rat hepatoma cells from hypoxia-induced apoptosis under conditions where ROS were not detectable [90,91]. A recently identified member of the Bcl-2 family, Nip3, has been shown to exert pro-apoptotic effects [92]. At present it is the only molecule of this family which is inducible by hypoxia.
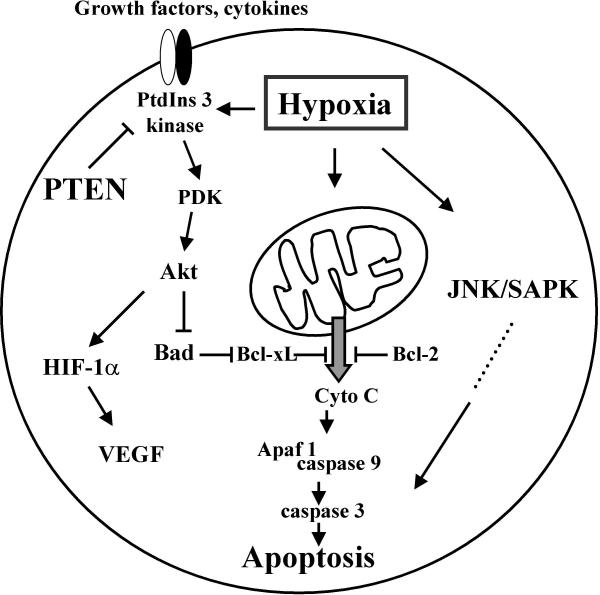
Figure 3.
Hypoxic activation of apoptosis pathways. Hypoxia activates intracellular signalling pathways involved in apoptosis and cell survival. One pathway of particular importance is hypoxia-induced mitochondrial membrane permability, leading to subsequent release of cytochrome C into the cytoplasm. Cytochrome C initiates the apoptosis cascade via activation of the apoptosis kinase Apaf-1, which in turn activates the caspase 9 apoptosis pathway. Hypoxia also activates JNK/SAPK signalling pathways which leads to apoptosis induction by an as yet unknown mechanism. The protein kinase Akt plays a central role in cell survival via induction of anti-apoptotic mechanisms, involving the anti-apoptotic function of Bcl-xL. Akt is also involved in hypoxia-induced HIF-dependent VEGF expression, a signalling cascade that can be inhibited by the tumor suppressor PTEN. PTEN exerts its negative regulatory effects via inhibition of phosphatidylinositol (3,4) and phosphatidylinositol (3,4,5) phosphorylation. Late-stage tumors often display mutated PTEN or show a complete loss of PTEN expression, which leads to a de-repression of the survival phosphatidylinositol (PtdIns) 3-kinase-Akt signalling pathway. PDK, PtdIns (3,4,5)P3-dependent kinase.
Although not directly linked to the caspase 9 signalling cascade the caspase 8 system might also be involved in apoptosis regulation under hypoxia. Recently, it could be shown that caspase 8 is involved in hypoxia-induced apoptosis in Jurkat cells [93]. In accordance with the latter paper we were able to show that hypoxia induced caspase 8 mRNA in melanoma cells (unpublished observation). In this respect it is of particular importance, that the cellular expression levels of caspase 8 might influence the sensitivity to apoptosis inducing agents. This was emphasized in a study demonstrating that neuroectodermal brain tumor cells lacking caspase 8 expression were resistant to TRAIL induced apoptosis. Treatment with a methyltransferase inhibitor restored caspase 8 expression and led to enhanced apoptosis sensitivity. Thus, caspase 8 expression levels might contribute to apoptosis sensitivity of tumor cells [94].
Recently, it could be shown that experimental deprivation of glucose induced apoptosis in myc-transformed fibroblasts [95]. Since glucose deprivation also occurs in the center of undervascularized and hypoxic tumors it might contribute to hypoxia-induced apoptosis in vivo. Furthermore, data from another group showed that not hypoxia itself but concomitant acidosis may be the main trigger for apoptosis under hypoxic conditions [96]. It could be demonstrated that in normal fibroblasts hypoxia-induced cell cycle arrest. In contrast, in oncogene transformed fibroblasts hypoxia induced apoptosis. The latter mechanism, however, required acidosis. If acidosis was removed from culture conditions by buffering, tumor cell viability and clonogenicity – of transformed fibroblasts – were both enhanced. Thus, hypoxia has not only a different impact on normal versus transformed cells, but its effects may also depend on the presence of acidosis in the tissue microenvironment. Further experiments have to address the question whether this phenomenon is cell-type specific.
It had been shown that p53 induction under severe hypoxia was HIF-1α dependent [97]. Thus, HIF-1α exerts a dual function by promoting tumor growth via the induction of angiogenesis, and, by promoting apoptosis via stabilization of the p53 protein. Under hypoxia the phosphorylation status of HIF-1α is a critical factor for the decision whether HIF may promote apoptosis or not. While dephosphorylated HIF-1α exerts pro-apoptotic effects under hypoxia its phosphorylated form does not [98]. The decision, which way the cells go was dependent on the severity of hypoxia [97].
The phosphatidylinositol (PtdIns) 3-kinase-Akt signalling pathway plays a central role in mediating signals derived from growth factors and cytokines that induce cell survival and proliferation (Fig. 3). Akt is a serine/threonine kinase which phosphorylates substrates leading to a decrease of the activity of pro-apoptotic molecules and an increase in the activity of anti-apoptotic molecules [for review, see [99,100]]. In particular, Akt promotes cell survival by preventing Bad from inhibiting the anti-apoptotic activity of Bcl-xL. Akt also inhibits the alteration of the mitochondrial membrane potential. Moreover, Akt induces NF-κB activation via phosphorylation of IκB kinase α, thereby increasing the expression of genes promoting cell survival. It also acts on cell cycle inhibitors and the well-known apoptosis molecule caspase 9. Recently, it could be shown that Akt signalling could be activated by hypoxia and prevented PC12 cells from hypoxia-induced apoptosis [101]. Moreover, HER-2/neu-overexpressing breast cancer cell lines were shown to be resistant to hypoxia-induced apoptosis and suppression of apoptosis could be reversed by PtdIns 3-kinase inhibitors such as LY294002 and wortmannin [102]. Thus, Akt could play an important role for apoptosis resistance under hypoxia.
PTEN, a recently discovered tumor suppressor gene has been shown to be mutated or lost in a variety of different tumors, in particular in tumors of advanced stage [103-105]. It could be shown that germline mutations in PTEN underly Cowden syndrome [106], a disease characterized by the occurence of multiple tumors in affected patients. Functionally, PTEN acts as a lipid phosphatase which dephosphorylates PtdIns lipids, such as PtdIns (4,5)P2 and PtdIns (3,4,5)P3. This leads to a disruption of PtdIns 3-kinase-Akt signalling (Fig. 3). It could be shown that this lipid phosphatase activity of PTEN is indeed important for its tumor suppressor function [107]. In the mentioned study reconstitution of wild type PTEN in the PTEN-deficient prostate cancer cell line LnCaP inhibited cell survival. Furthermore, transfection of LnCaP cells with constitutively active Akt reverted the phenotype of PTEN deficient cells.
In a recent study it could be shown that overexpression of wildtype PTEN suppressed hypoxia-mediated activation of Akt in glioblastoma cell lines [108]. Although without influence on the rate of apoptotic cells under hypoxia, reconstitution of wildtype PTEN in PTEN-deficient glioblastoma cells downregulated angiogenesis factors such as VEGF (Fig. 3). This process involved the transcription factor HIF-1α (Fig. 3). Thus, PTEN expression negatively interfered with hypoxia-induced angiogenesis factor expression in glioblastoma cells. Since loss of PTEN is a common feature in late-stage tumors enhanced angiogenesis factor production under hypoxia might indirectly promote cell survival under the adverse conditions of the tumor microenvironment.
Recently, the oncoprotein Mdm2 had been identified as another target for Akt [for review, see [100]]. Activation of Akt signalling pathway promotes nuclear entry of Mdm2. There, it interacts with p53 tumor suppressor protein and targets p53 for proteasomal degradation. PTEN had been shown to inhibit movement of Mdm2 into the nucleus and thereby inhibited p53 proteasomal degradation, rendering cells susceptible for apoptosis inducing stimuli. Moreover, p53 binding motifs have been identified in the PTEN promoter [109]. In the latter study p53 induced PTEN transcription and cellular levels of PTEN protein, thus providing a pro-apoptotic p53-PTEN pathway. The role of hypoxia in this regulatory pathway has also been addressed. It could be demonstrated that hypoxia suppressed Mdm2 expression and thereby enhanced p53 expression [110]. Interestingly, however, in a recent study the transcriptional activity of stabilized p53 induced by deferoxamine mesylate, which mimics hypoxia, was lost in all the tested tumor cell lines [111]. Thus, hypoxia-induced stabilization of p53 does not necessarily relate to p53 function.
Taken together, hypoxia mediated apoptosis utilizes different intracellular pathways involving apoptosis and cell survival molecules such as cytochrome C, members of the Bcl-2 family, Akt and PTEN.
Conclusions
In the present report the current knowledge about hypoxia-induced signal transduction, gene regulation, angiogenesis factor production and apoptosis regulation is summarized with a special emphasis on molecular mechanisms. Many of the presented findings demonstrate that hypoxia critically interferes with tumor progression and tumor aggressiveness. As a consequence, a series of studies are currently underway targeting molecular processes related to tissue hypoxia in tumor cells. Some of the recent studies have already provided evidence that tumor growth might be influenced via interference with transcription factors, such as HIF-1α. Since the molecular mechanisms are becoming more and more clear future therapies may find new target molecules in tumor cells, finally leading to a more efficient treatment of aggressive and metastasizing tumors.
Authors' contributions
Most of the experimental work cited from the authors' laboratories were carried out by MK. SMI contributed to this paper mainly by writing and in the overall conception of the paper. Both authors read and approved the final manuscript.
List of abbreviations used
AP-1, activator protein-1; ARNT, Aryl hydrocarbon receptor nuclear translocator; CBP, CREB binding protein; ERK, extracelluar signal-regulated kinase; FIH, factor inhibiting HIF-1α; H, hypoxia; HIF-1α, hypoxia inducible factor-1α; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MAPKKK, mitogen-activated protein kinase kinase kinase; MEK, MAPK/ERK kinase; NF-κB, nuclear factor κB; N, normoxia; PtdIns 3-kinase, phosphatidylinositol 3-kinase; PDK, PtdIns (3,4,5)P3-dependent kinase; PTEN, Phosphatase and Tensin homolog deleted on chromosome Ten; SAPK, stress-activated protein kinase; TRAIL, TNF-related apoptosis inducing ligand; VHL, von Hippel Lindau protein.
Contributor Information
Manfred Kunz, Email: manfred.kunz@med.uni-rostock.de.
Saleh M Ibrahim, Email: saleh.ibrahim@med.uni-rostock.de.
References
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: High resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nature Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Coleman CN, Mitchell JB, Camphausen K. Tumor hypoxia: chicken, egg, or a piece of the farm? J Clin Oncol. 2002;20:610–615. doi: 10.1200/JCO.2002.20.3.610. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Webster KA. Hypoxia/reoxygenation stimulates Jun kinase activity through redox signaling in rat cardiac myocytes. Circ Res. 1997;80:336–344. doi: 10.1161/01.res.80.3.336. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Mendonca HL, Calaoagan JM, Knapp AM, Giaccia AJ, Stork PJS. Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironment. J Biol Chem. 1999;274:12890–12897. doi: 10.1074/jbc.274.18.12890. [DOI] [PubMed] [Google Scholar]
- Kunz M, Ibrahim S, Koczan D, Thiesen HJ, Koehler HJ, Acker T, Plate KH, Ludwig S, Rapp UR, Broecker EB, et al. Activation of c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) is critical for hypoxia-induced apoptosis of human malignant melanoma. Cell Growth Differ. 2001;12:137–145. [PubMed] [Google Scholar]
- Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/S0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Copp MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/S0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Daum G, Eisenmann-Tappe I, Fries HW, Troppmair J, Rapp UR. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL. MEKKs, GCKs, MLKs, PAKs, TAKs, and Tpls: upstream regulators of the c-Jun amino-terminal kinases. Curr Opin Gen Dev. 1997;7:67–74. doi: 10.1016/S0959-437X(97)80111-6. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Zhang HL, Steinberg SF. Hypoxia alters the subcellular distribution of the protein kinase C isoforms in neonatal rat ventricular myocytes. J Clin Invest. 1997;99:55–61. doi: 10.1172/JCI119133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Bloss G, Gillitzer R, Gross G, Goebeler M, Rapp UR, Ludwig S. Hypoxia/reoxygenation induction of monocyte chemoattractant protein-1 in melanoma cells: involvement of nuclear factor-kappaB, stimulatory protein-1 transcription factors and mitogen-activated protein kinase pathways. Biochem J. 2002;366:299–306. doi: 10.1042/BJ20011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/S0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Wojnowski L, Zimmer AM, Beck TW, Hahn H, Bernal R, Rapp UR, Zimmer A. Endothelial apoptosis in Braf-deficient mice. Nat Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and response to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/S0955-0674(99)00071-X. [DOI] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Sheta EA, Trout H, Gildea JJ, Harding MA, Theodorescu D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene. 2001;20:7624–7634. doi: 10.1038/sj.onc.1204972. [DOI] [PubMed] [Google Scholar]
- Berra E, Milanini J, Richard DE, Le Gall M, Vinals F, Gothie E, Roux D, Pages G, Pouyssegur J. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60:1171–1178. doi: 10.1016/S0006-2952(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1, O(2), and the 3 PHDs. 2001. How animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O 2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–1686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E, Pages G, Pouyssegur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000;19:139–145. doi: 10.1023/a:1026506011458. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr How oxygen makes its presence felt. Genes Dev. 2002;16:1441–1445. doi: 10.1101/gad.1003602. [DOI] [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419X(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Rupec RA, Baeuerle PA. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-κB. Eur J Biochem. 1995;234:632–640. doi: 10.1111/j.1432-1033.1995.632_b.x. [DOI] [PubMed] [Google Scholar]
- Yao KS, Xanthoudakis S, Curran T, O'Dwyer PJ. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Mol Cell Biol. 1994;14:5997–6003. doi: 10.1128/mcb.14.9.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, van Meir EG. Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma. Oncogene. 1999;18:1447–1456. doi: 10.1038/sj.onc.1202424. [DOI] [PubMed] [Google Scholar]
- Kunz M, Hartmann A, Flory E, Toksoy A, Koczan D, Thiesen HJ, Mukaida N, Neumann M, Rapp UR, Bröcker EB, Gillitzer R. Anoxia-induced up-regulation of interleukin-8 in human malignant melanoma. A potential mechanism for high tumor aggressiveness. Am J Pathol. 1999;155:753–763. doi: 10.1016/S0002-9440(10)65174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels C, Minet E, Michel G, Mottet D, Piret JP, Raes M. HIF-1 and AP-1 cooperate to increase gene expression in hypoxia: role of MAP kinases. IUBMB Life. 2001;52:49–53. doi: 10.1080/15216540252774766. [DOI] [PubMed] [Google Scholar]
- Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, et al. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. NF-κB signalling. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327:419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Calaoagan JM, Gustafson-Brown C, Knapp AM, Li GC, Mendonca HL, Ryan HE, Wang Z, Johnson RS. The response of c-jun/AP-1 to chronic hypoxia is hypoxia-inducible factor 1 alpha dependent. Mol Cell Biol. 2002;22:2515–2523. doi: 10.1128/MCB.22.8.2515-2523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfranca A, Gutierrez MD, Vara A, Aragones J, Vidal F, Landazuri MO. c-Jun and hypoxia-inducible factor 1 functionally cooperate in hypoxia-induced gene transcription. Mol Cell Biol. 2002;22:12–22. doi: 10.1128/MCB.22.1.12-22.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM. Sp1 and the subfamily of zinc finger proteins with guanine-rich binding sites. Proc Natl Acad Sci U S A. 1992;89:11109–11110. doi: 10.1073/pnas.89.23.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Ji YS, Schmedtje JF., Jr Sp1 increases expression of cyclooxygenase-2 in hypoxic vascular endothelium. Implications for the mechanisms of aortic aneurysm and heart failure. J Biol Chem. 2000;275:24583–24589. doi: 10.1074/jbc.M003894200. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- Rak J, Yu JL, Klement G, Kerbel RS. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J Investig Dermatol Symp Proc. 2000;5:24–33. doi: 10.1046/j.1087-0024.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Singh RK, Gutman M, Radinsky R, Bucana CD, Fiedler IJ. Expression of interleukin-8 correlates withe metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L, Joseph-Silverstein J, et al. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci U S A. 1995;92:4606–4610. doi: 10.1073/pnas.92.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Kunz M, Köstlin S, Gillitzer R, Toksoy A, Bröcker EB, Klein CE. Hypoxia-induced up-regulation of angiogenin in human malignant melanoma. Cancer Res. 1999;59:1578–1583. [PubMed] [Google Scholar]
- Xu L, Xie K, Mukaida N, Matsushima K, Fidler IJ. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res. 1999;59:5822–5829. [PubMed] [Google Scholar]
- Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–887. [PubMed] [Google Scholar]
- Lal A, Peters H, St Croix B, Haroon ZA, Dewhirst MW, Strausberg RL, Kaanders JH, van der Kogel AJ, Riggins GJ. Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst. 2001;93:1337–1343. doi: 10.1093/jnci/93.17.1337. [DOI] [PubMed] [Google Scholar]
- Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;8:1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc Natl Acad Sci U S A. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KA, Fett JW, French TC, Key ME, Vallee BL. Angiogenin antagonists prevent tumor growth in vivo. Proc Natl Acad Sci USA. 1995;92:442–446. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- O'Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Hornby AE, Lakins J, Lupu R. Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies. Cancer Res. 2000;60:5603–5607. [PubMed] [Google Scholar]
- Shimo T, Kubota S, Kondo S, Nakanishi T, Sasaki A, Mese H, Matsumura T, Takigawa M. Connective tissue growth factor as a major angiogenic agent that is induced by hypoxia in a human breast cancer cell line. Cancer Lett. 2001;174:57–64. doi: 10.1016/S0304-3835(01)00683-8. [DOI] [PubMed] [Google Scholar]
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- Barnhill RL, Piepkorn MW, Cochran AJ, Flynn E, Karaoli T, Folkman J. Tumor vascularity, proliferation, and apoptosis in human melanoma micrometastases and macrometastases. Arch Dermatol. 1998;134:991–994. doi: 10.1001/archderm.134.8.991. [DOI] [PubMed] [Google Scholar]
- Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17:3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene. 1998;17:3401–3415. doi: 10.1038/sj.onc.1202590. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Lin Z, Vincenz C, Brosius FC 3rd. Hypoxia induces apoptosis via two independent pathways in Jurkat cells: differential regulation by glucose. Am J Physiol Cell Physiol. 2001;281:C1596–C1603. doi: 10.1152/ajpcell.2001.281.5.C1596. [DOI] [PubMed] [Google Scholar]
- Grotzer MA, Eggert A, Zuzak TJ, Janss AJ, Marwaha S, Wiewrodt BR, Ikegaki N, Brodeur GM, Phillips PC. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene. 2000;19:4604–4610. doi: 10.1038/sj.onc.1203816. [DOI] [PubMed] [Google Scholar]
- Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci U S A. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Tomida A, Tsuruo T. Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene. 2001;20:5779–5788. doi: 10.1038/sj.onc.1204742. [DOI] [PubMed] [Google Scholar]
- Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/S0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–467. doi: 10.1016/S0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- Bacus SS, Altomare DA, Lyass L, Chin DM, Farrell MP, Gurova K, Gudkov A, Testa JR. AKT2 is frequently upregulated in HER-2/neu-positive breast cancers and may contribute to tumor aggressiveness by enhancing cell survival. Oncogene. 2002;21:3532–3540. doi: 10.1038/sj.onc.1205438. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Rasheed BK, Stenzel TT, McLendon RE, Parsons R, Friedman AH, Friedman HS, Bigner DD, Bigner SH. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res. 1997;57:4187–4190. [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- Alarcon R, Koumenis C, Geyer RK, Maki CG, Giaccia AJ. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res. 1999;59:6046–6051. [PubMed] [Google Scholar]
- Ashcroft M, Taya Y, Vousden KH. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/MCB.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]