Abstract
At least 16 proteins are thought to be involved in forming the enteropathogenic Escherichia coli (EPEC) type III translocation apparatus which delivers virulence factors into host cells, yet their function and location have not been determined. A biochemical analysis was performed on three components: EscN, a predicted cytoplasmic ATPase; EscV, a predicted inner membrane protein; and EscC, a predicted outer membrane secretin. Wild-type EPEC and mutants constructed in these genes were fractionated by lysozyme treatment, ultracentrifugation, and selective detergent extraction. Fractionation revealed that the type III effectors Tir and EspB required a complete type III apparatus for any degree of export by EPEC, suggesting a continuous channel. Epitope-tagged EscC, EscV, and EscN were localized by fractionation, confirming computer modeling predictions for their location. Transcomplementation experiments revealed that localization of EscV and EscN was unaffected by mutations in other examined type III components. Remarkably, localization of EscC was altered in escV or escN mutants, where EscC accumulated in the periplasm. EscC was correctly localized in the escF needle component mutant, indicating that secretin localization is independent of needle formation. These data indicate that, contrary to previous indications, correct insertion and function of EscC secretin in the outer membrane depends not only on the sec-dependent secretion pathway but also on other type III apparatus components.
The delivery of virulence factors directly into host cells to interfere with and alter host processes is a crucial step in bacterial virulence for several significant animal and plant pathogens (56, 64). The type III secretion system (TTSS) facilitates delivery of many bacterial effectors directly from the cytoplasm of gram negative bacteria into host cells, thereby crossing the bacterial inner membrane, peptidoglycan, and outer membrane, as well as the host plasma membrane.
Enteropathogenic Escherichia coli (EPEC) is a human pathogen responsible for outbreaks of diarrhea in both developing and developed countries (46). During infection, EPEC adheres to intestinal epithelial cells through the binding of the outer membrane protein, intimin, to its receptor in the host. EPEC inserts a receptor for intimin, Tir (translocated intimin receptor), into the host cell membrane, where it becomes tyrosine phosphorylated (10, 31). Intimin binding induces the rearrangement of the host cytoskeletal structure to form attaching and effacing lesions, which are characterized by degradation of the brush border microvilli and formation of actin-rich pedestals upon which the bacteria reside (65). While the mechanism of Tir insertion is not known, it is mediated by EPEC's TTSS and secreted proteins EspA, EspB, and EspD (31). EspA forms a filamentous organelle located on the bacterial surface that is postulated to act as a channel for the type III system to deliver proteins inside the host cell (14, 34). Recently, it has been demonstrated by electron microscopy that EspA forms the sheath or coat of the needle and determines its length and that the needle is composed of EscF (7, 58, 69). EspB and EspD are translocated into the host cell membrane, with EspB also found in the cytoplasm, and together are thought to form the translocation pore (27, 36, 63, 67, 68, 71). Furthermore, EspA and EspB have been shown to interact by a number of binding assays (24).
Components of the type III secretion apparatus have been identified by creating mutations and testing for a lack of secretion and a decrease in type III secretion-dependent phenotypes (26, 43). EscN is thought to be the energizer of the secretion machinery, sharing homology with the F0F1 ATPase and having a putative ATP-binding site. While EscN has not been studied, the homologous proteins YscN from Yersinia spp. and InvC from Salmonella enterica serovar Typhimurium have been shown to have ATPase activity, and mutations in the catalytic domain cause a loss of secretion (15, 70). Type III EscN homologues are predicted to be located in the cytoplasm, where they interact with membrane-bound components of the type III apparatus, thereby energizing the system (26). The energy requirements of type III secretion and translocation are not well understood.
Many of the proteins involved in forming the TTSS have been localized or are predicted to be inner membrane proteins with various numbers of transmembrane domains. YscV (LcrD), an EscV homologue in Yersinia species, has been studied previously and contains eight transmembrane domains and a large cytoplasmic carboxy-terminal domain (51, 53). However, EscV has not been examined, nor has a function been ascribed to this family of proteins.
Homologues of EscC (e.g., YscC, InvG, and HrcC) are the only components of the type III apparatus that are clearly found in the outer membrane (5, 12, 35, 54). EscC belongs to a family of proteins (secretins) which are involved in transporting large molecules across the outer membrane, probably by forming a channel (26). This family is divided into four classes: (i) type III secretion pathway, (ii) type II general secretion pathway (PulD), (iii) extrusion and assembly of filamentous phage, and (iv) export of pilus subunits in the assembly of type IV pili (19, 26, 42). The EscC homologue YscC of Yersinia enterocolitica has been shown to form a ring-shaped oligomeric complex in the outer membrane with a diameter of approximately 20 nm (35). Similar results have been seen with InvG of Salmonella serovar Typhimurium (5). The secretin PulD from Klebsiella oxytoca has been shown by electron microscopy to be composed of two stacked rings that surround a central channel, where the N-terminal domain folds to occlude the channel (49). Interestingly, PulD forms a complex in the outer membrane which is dependent on the lipoprotein PulS (22, 23, 48).
There are considerable similarities between the flagellar secretion system (or flagellar type III export system) and the virulence-associated TTSS. The structure of the type III system is very reminiscent of flagellar basal bodies, which is not surprising, considering that 10 type III genes encoding cytoplasmic and inner membrane proteins are similar to flagellar genes (1, 41, 52). The outer membrane secretin EscC does not have a flagellar homologue, and there are no obvious direct flagellar homologues for EspA and EscF.
With the availability of increased numbers of genomic sequences and computer modeling, proteins are often ascribed functions through similarities to other proteins. This has been the case for the components of the TTSS, but this does not eliminate the need to confirm these predictions experimentally. In this study, we experimentally determined the locations of three EPEC type III apparatus proteins—EscN, EscV, and EscC—and investigated their functions. In protein transport systems of mitochondrial import, type II secretion, and flagellar export, mutants have been used to elucidate the pathways by examining where substrates accumulate in intermediate positions (25, 38, 44, 50, 55). We used a similar approach to biochemically examine EPEC's TTSS by investigating the locations of these type III components and by determining if mutations in one apparatus component affected the localization of other components or of the type III secreted proteins Tir and EspB.
MATERIALS AND METHODS
Bacterial strains.
The wild-type EPEC strain E2348/69 (streptomycin resistant) was used in this study. Strains were grown in Luria-Bertani broth supplemented with appropriate antibiotics as standing overnight cultures at 37°C.
Construction of nonpolar mutants. (i) escC deletion mutant.
As detailed by de Grado et al. (9), oligonucleotides ESCC-01F (XhoI restriction site) and ESCC-02R (SacI restriction site) were used to amplify escC with chromosomal DNA from EPEC E2348/69. The amplification product was cloned into pCR2.1 TOPO (Invitrogen), generating pCR-escC. Primers ESCC-03R and ESCC-04F were used to create an in-frame deletion of the escC gene in pCR-escC by using inverse PCR amplification. Both oligonucleotides ESCC-03R and ESCC-04F introduced an MluI restriction site. The 2,049-bp SacI-XhoI escC deletion fragment was cloned into the positive-selection suicide vector pCVD442 (13), which had been digested with SacI-SalI. The resulting plasmid, pCVD442-ΔescC, was used to construct the escC deletion mutant in EPEC E2348/69 (streptomycin resistant) by allelic exchange as described previously (13), generating the EPEC ΔescC strain (9).
(ii) escV deletion mutant.
The EPEC ΔescV strain was constructed as described above by using primers ESCV-05F, ESCV-06R, ESCV-07R, and ESCV-08F.
(iii) escN deletion mutant.
The EPEC ΔescN strain was constructed as described above by using primers ESCN-09F, ESCN-10R, ESCN-13R, and ESCN-14F, except that a BstEII site was introduced.
Construction of plasmids expressing escC, escV, escN, and their HSV-tagged versions.
By using the appropriate primer pairs (Table 1), esc genes were amplified from EPEC E2348/69, introducing BamHI and SalI restriction sites. The amplified products were cloned into the BamHI-SalI sites in pACYC184 (New England Biolabs), leaving the genes under the control of the tetracycline resistance gene promoter, thus generating pEscC, pEscV, and pEscN. By using the appropriate primer pairs (Table 1), esc genes were amplified from EPEC E2348/60, introducing BamHI and NheI restriction sites. The amplified products were cloned into the BamHI and NheI sites in pET27b(+) (Novagen) in order to be tagged with herpes simplex virus (HSV), creating an in-frame translational fusion on the 3′ end of the gene (corresponding to the C terminus of the protein). Esc-HSV-tagged genes were amplified from the pET27b derivatives and cloned into the BamHI-SalI sites in pACYC184, leaving the genes under the control of the tetracycline resistance gene promoter, thus generating pEscC-HSV, pEscV-HSV, and pEscN-HSV.
TABLE 1.
Primers used in this study (5′ → 3′)
| Construct and primera | Sequence |
|---|---|
| escC deletion mutant | |
| ESCC-01F | GTT AAC CTC GAG GCG GTT CCG ATA G |
| ESCC-02R | GAT GCG AGC TCT GTT GCT ATC CAA TG |
| ESCC-03R | GGC GAC GCG TGT ATA CCG CTG TTA AGC GAC ATT CC |
| ESCC-04F | GGC GAC GCG TCA TTA CAC AAT TCG TCC TAT ATC AG |
| escV deletion mutant | |
| ESCV-05F | CGG GAT GAG CTC ATC AAC GAC ATT TG |
| ESCV-06R | GGG AAA TGG TCG ACC TCT GCA AGT TCT C |
| ESCV-07R | GGC GAC GCG TGA GTT TAT TCA TGA TGT CAT CCT G |
| ESCV-08F | GGC GAC GCG TGT CCC CGT GCT CTC TTT TCA GGA G |
| escN deletion mutant | |
| ESCN-09F | CTA TGG GAG CTC ATT TGT CTA ATA G |
| ESCN-10R | GGT ATC AGT CGA CTT TCA CTA TAC G |
| ESCN-13R | GGC GGG TCA CCA ATA CAG AAT CAT GCT CTG AAA TC |
| ESCN-14F | GGC GGG TCA CCC AAC AAA GCA CCA AAG ATA TCA GTA G |
| pEscC | |
| ESCC-B-15 | CGC GGA TCC TCT AAG ATA TAG GAC GAA TTA TG |
| ESCC-S-16 | CGC CGT CGA CTT ATT CGC TAG ATG CAG ATT TTA TCG G |
| pEscC-HSV | |
| ESCC-B-15 | CGC GGA TCC TCT AAG ATA TAG GAC GAA TTA TG |
| ESCC-29 | CGC GCT AGC TTC GCT AGA TTT TAT CGG |
| HSV-30 | GC GTC GAC TTA ATC CTC GGG GTC TTC CGG |
| pEscV | |
| ESCV-B-17 | GGC GGA TCC TCT AAG AGC GCG TTC GCA GGA TG |
| ESCV-S-18 | ACG CGT CGA CTC ATG CTC TGA AAT CAT TTA CCG |
| pEscV-HSV | |
| ESCV-B-17 | GGC GGA TCC TCT AAG AGC GCG TTC GCA GGA TG |
| ESCV-31 | CGC GCT AGC TGC TCT GAA ATC ATT TAC CG |
| HSV-30 | GC GTC GAC TTA ATC CTC GGG GTC TTC CGG |
| pEscN | |
| ESCN-B-19 | CGC GGA TCC TCT AAG GGA ATA ATA TCG AAC TTA AAG |
| ESCN-S-20 | ACG CGT CGA CTC AGG CAA CCA CTT TGA ATA GGC |
| pEscN-HSV | |
| ESCN-B-19 | CGC GGA TCC TCT AAG GGA ATA ATA TCG AAC TTA AAG |
| ESCN-32 | CGC GCT AGC GGC AAC CAC TTT GAA TAG GC |
| HSV-30 | GC GTC GAC TTA ATC CTC GGG GTC TTC CGG |
Primers are listed under mutants or plasmids in whose construction they were used.
Bacterial fractionation.
Bacterial cell fractionation was based on previously described procedures (17, 28, 47). Briefly, EPEC from an overnight standing culture was subcultured 1/50 in 100 ml of minimal essential medium for 3 h at 37°C under 5% CO2. The culture was harvested, washed in phosphate-buffered saline, and resuspended in 1 ml of 50 mM Tris (pH 7)-20% sucrose with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, 1 μM pepstatin, 10 μg of aprotinin/ml). Cells were treated with 40 μl of 0.25 M EDTA-0.25 mg of lysozyme/ml (final concentrations, 10 mM and 10 μg/ml, respectively) for 10 min at room temperature. All subsequent steps were carried out at 4°C. The periplasmic fraction was isolated from the rest of the cells by centrifugation (at 8,000 × g for 10 min). The pellet was resuspended in 1 ml of sonication buffer (10 mM Tris-HCl [pH 7] and protease inhibitors) and sonicated three times for 15 s each time (Fisher Sonicator, amplitude 2.5). Unbroken bacteria were removed by centrifugation (at 16,000 × g for 2 min), and the cleared supernatant containing cytoplasmic proteins and inner and outer membranes was removed and centrifuged (in a Beckman TLA 100 Ultracentrifuge with a TLA100.3 rotor) for 1 h at 50,000 × g to pellet the membranes. The supernatant containing the cytoplasmic fraction was removed; the membrane pellet was washed with sonication buffer, resuspended in 0.1 ml sonication buffer with 0.5% N-lauroylsarcosine, which selectively solubilizes the inner membrane, and centrifuged (at 50,000 × g for 1 h); the supernatant containing the inner membrane fraction was removed; and the outer membrane pellet was washed in sonication buffer with 0.5% N-lauroylsarcosine. The final pellet was resuspended in 0.1 ml of sonication buffer with 0.5% N-lauroylsarcosine and 0.1% sodium dodecyl sulfate (SDS). The protein content of all samples was determined by using the Bio-Rad protein assay (periplasmic fraction) or the bicinchoninic acid (BCA) protein assay (Sigma) (other fractions) before addition of SDS sample buffer with β-mercaptoethanol. The above fractionation protocol yielded approximately 0.33 mg of protein in the periplasmic fraction, 3.3 mg of protein in the cytoplasmic fraction, and 0.1 mg of protein in each of the inner membrane and outer membrane fractions.
Phenol treatment.
To reduce the EscC complex seen in the outer membrane fraction to the monomer, outer membrane pellets obtained by the procedure described above were resuspended in 0.1 ml of sonication buffer and treated with phenol as described previously (21). Briefly, outer membrane samples were extracted with 0.1 ml of buffer-saturated phenol (Gibco BRL) at 70°C for 10 min, cooled to 4°C, and centrifuged at 5,000 × g for 10 min. The upper aqueous layer (which had no detectable protein) was discarded. The interface and the phenol layer were treated with 0.1 ml of distilled water at 70°C again, and the aqueous phase was discarded after centrifugation as described above. The proteins in the organic phase were precipitated with 2 volumes of acetone at 4°C overnight and then pelleted by centrifugation (at 16,000 × g for 20 min). The pellet was washed with acetone, and once with 2 volumes of ether, and was collected by centrifugation. The pellet was air dried before being resuspended in 0.1 ml of sonication buffer with 0.5% N-lauroylsarcosine and 0.1% SDS. The protein content of all samples was determined by using a BCA protein assay before addition of SDS sample buffer with β-mercaptoethanol.
Immunoblotting.
Samples (10 μg) were subjected to SDS-PAGE and transferred to nitrocellulose filters (pure nitrocellulose; pore size, 0.45 μm; Bio-Rad). Blots were blocked overnight at 4°C in 5% (wt/vol) skim milk-TTBS (0.1% Tween 20-Tris-buffered saline), incubated first with the primary antibody diluted in 1% skim milk-TTBS for 1 h at room temperature and then with the secondary antibody diluted in 1% skim milk-TTBS for 1 h at room temperature, and detected with the ECL reagent (Amersham). Primary antibodies were a mouse anti-Tir monoclonal antibody, clone 2A8 (8), diluted 1/1,000 and preabsorbed with a fixed EPEC tir mutant; a mouse anti-EspB monclonal antibody, clone 2A11, diluted 1/100; mouse anti-DnaK (Stressgen), diluted 1/1,000; rabbit anti-maltose binding protein (anti-MBP) (New England Biolabs), diluted 1/3,000; rabbit anti-intimin, diluted 1/3,000, and rabbit anti-Etk (diluted 1/3,000) (kind gifts from I. Rosenshine); and mouse anti-HSV (Novagen), diluted 1/1,000. Secondary antibodies were a horseradish peroxidase-conjugated goat anti-mouse antibody (Jackson ImmunoResearch), diluted 1/5,000, and a horseradish peroxidase-conjugated goat anti-rabbit antibody (Sigma), diluted 1/5,000.
RESULTS
EscC, EscV, and EscN are required for functional type III translocation by EPEC.
As an initial examination of the type III secretion pathway, and to investigate the function of three type III apparatus proteins that are predicted to be located in different compartments of EPEC and that are highly conserved in other TTSSs, nonpolar deletion mutants were constructed for the genes encoding EscN, EscV, and EscC. We assayed for functional type III translocation by EPEC by examining whether pedestals formed in HeLa cells infected with wild-type EPEC or the mutants by immunofluorescence microscopy. While wild-type EPEC formed microcolonies and accumulated phosphotyrosine-containing proteins and actin under the bacteria, the escC, escV, and escN mutants did not form pedestals (data not shown). Lack of secretion of Esps and Tir, and lack of Tir translocation into HeLa cells, also confirmed the essential role of these three proteins in the functionality of the type III apparatus (18) (data not shown).
To demonstrate that the mutations were nonpolar, the three mutants were rescued with the appropriate esc gene. escC, escV, and escN were amplified and cloned either untagged or HSV tagged on the 3′ end (corresponding to the C terminus) into pACYC184. The resulting plasmids carrying either the untagged or the HSV-tagged esc genes (Table 1) were transformed into the corresponding mutants, which were then able to form pedestals on HeLa cells, as demonstrated by immunofluorescence microscopy (data not shown). Therefore, the mutations of the escC, escV, and escN genes were nonpolar and could be complemented, and the epitope tags did not interfere with the function of these three proteins.
Tir and EspB accumulate in the cytoplasm of type III mutants.
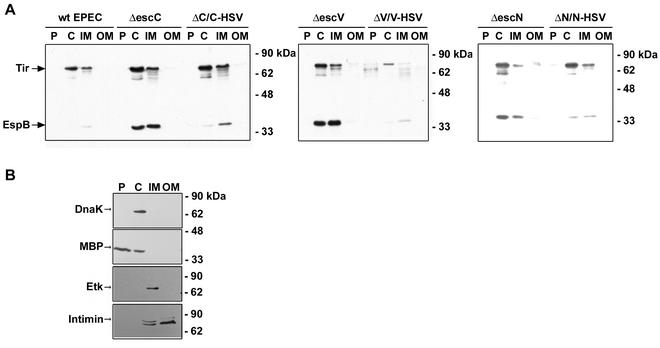
In order to investigate the pathway of secretion through the type III apparatus, Tir and EspB distribution was examined in wild-type EPEC and type III mutants. Bacteria were fractionated into periplasmic, cytoplasmic, and inner and outer membrane fractions by lysozyme treatment, ultracentrifugation, and selective detergent extraction with sarcosyl, respectively, as detailed in Materials and Methods. While multiple methods exist for fractionating bacteria, this method was chosen because it is well established (17, 47) and, perhaps more importantly, has previously been used successfully to fractionate EPEC (28). The sarcosyl method takes advantage of the differing lipid properties of the inner and outer membranes. Sarcosyl has the same charge density as lipopolysaccharide (LPS), and it is thought that because it mimics LPS, it does not solubilize the LPS-based outer membrane (47). As a control for clean bacterial fractionation, samples (10 μg of protein of each fraction) were probed with anti-DnaK, anti-Etk, anti-intimin, and anti-MBP (Fig. 1B). DnaK is an abundant heat shock protein that functions as a chaperone inside the bacterial cytoplasm (40) and was found in the cytoplasmic fraction. Etk is an E. coli inner membrane tyrosine kinase (28) and was found in the inner membrane fraction. Intimin is the outer membrane ligand in EPEC (29) and is subject to N-terminal processing. As expected, the processed form was found in the outer membrane and the preprocessed form and a small amount of the processed form were found in the inner membrane. MBP is a periplasmic protein (30) and was found primarily in the periplasmic fraction but was also detected in the cytoplasmic fraction. Other methods for isolating clean periplasm, including freeze-thawing, various concentrations of lysozyme, and osmotic shock, were explored, but all methods resulted in significant contamination of the periplasmic fraction with cytoplasmic proteins (data not shown). Because our method resulted in a noncontaminated periplasmic fraction, it was appropriate for the purposes of this study. While the marker proteins were localized correctly by using this bacterial fractionation technique, this does not necessarily indicate that the type III apparatus and associated proteins would fractionate as cleanly, since it potentially spans both membranes. Sucrose density and flotation gradients were not successful at separating the inner and outer membranes (data not shown). The sarcosyl treatment released predicted inner membrane components of the TTSS (see the next section) and was thus used extensively for this study.
FIG. 1.
Tir and EspB accumulate in the cytoplasm of type III mutants. Bacteria were fractionated into periplasmic (P), cytoplasmic (C), and inner membrane (IM) and outer membrane (OM) fractions by lysozyme treatment, ultracentrifugation, and selective detergent extraction, respectively. Samples (10 μg of each fraction) were resolved by SDS-10% PAGE and transferred to a nitrocellulose filter. (A) Western blots were probed with an anti-Tir or anti-EspB antiserum. (B) Western blots were probed with an anti-DnaK, anti-MBP, anti-Etk, or anti-intimin antiserum to monitor for contamination of fractions.
In wild type EPEC, Tir and small amounts of EspB were detected in the cytoplasmic and inner membrane fractions (Fig. 1A), but most was secreted into the culture medium (data not shown). All three type III mutants had greater amounts of Tir and EspB detected in the cytoplasmic and inner membrane fractions than wild-type EPEC. All three mutants showed the same pattern of Tir and EspB distribution. The three mutants were rescued to give approximately a wild-type EPEC distribution of Tir and EspB when complemented in trans with the appropriate untagged (data not shown) or HSV-tagged (Fig. 1A) esc gene. The differences are likely due to the unbalanced expression of the genes that is driven by the tetracycline promoter of the plasmid, which uncouples the production of these proteins from the rest of the type III apparatus.
Localization of EscC, EscV, and EscN.
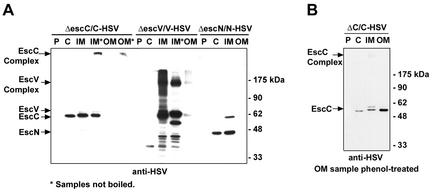
We next examined the location of EscC, EscV, and EscN by using the bacterial fractionation method and detecting the C-terminal HSV tag. EscC is predicted to be a 56-kDa preprotein with a putative signal sequence that has a cleavage site at amino acid 19, which would result in a 54-kDa outer membrane protein upon export by the sec-dependent secretion pathway. EscC-HSV was detected as a 60-kDa protein in the cytoplasmic and inner membrane fractions (Fig. 2A). A faint band was detectable above the 60-kDa band in the inner membrane fraction and the cytoplasm (at longer exposures); this most likely corresponds to the preprocessed form. Notably, we did not detect EscC in the outer membrane fraction. We hypothesized that boiling of the sample may have led to aggregation and precipitation of EscC from this fraction, as has been observed for other membrane proteins (66). When the samples were not boiled prior to electrophoresis, a high-molecular-weight form of EscC was detectable by Western blotting in the stacking gel in the inner membrane and outer membrane fractions. The high-molecular-weight complex in the outer membrane can be dissociated by phenol treatment to yield monomeric EscC (Fig. 2B). These results are analogous to those obtained with other EscC homologues (12, 22, 35, 54).
FIG. 2.
Localization of EscC, EscV, and EscN. Bacteria were fractionated into periplasmic (P), cytoplasmic (C), and inner membrane (IM) and outer membrane (OM) fractions by lysozyme treatment, ultracentrifugation, and selective detergent extraction, respectively. Samples (10 μg of each fraction) were resolved by SDS-10% PAGE, transferred to a nitrocellulose filter, and probed with anti-HSV antisera. (A) Asterisks indicate samples that were not boiled prior to electrophoresis. (B) The outer membrane fraction was treated with phenol prior to addition of SDS sample buffer.
EscV is predicted to be a 75-kDa preprotein with a putative sec-dependent signal sequence, cleavable at residue 28, yielding a 72-kDa inner membrane protein with 7 predicted transmembrane domains. Virtually all of the EscV-HSV was found in the inner membrane fraction (Fig. 2A). The major band of EscV-HSV had an apparent molecular mass of 64 kDa in the inner membrane, although a smear of protein was detected at higher molecular weights, suggesting that EscV forms SDS-resistant complexes. Smearing was reduced by not boiling the samples prior to electrophoresis (Fig. 2A).
EscN is predicted to be a 49-kDa cytoplasmic protein, with no signal sequence. EscN-HSV was detected at the predicted molecular mass in both the cytoplasmic and inner membrane fractions (Fig. 2A). Since EscN does not have any predicted transmembrane domains, it is unlikely to be a membrane protein. Furthermore, EscN is not found in the periplasmic fraction, suggesting an association with the cytoplasmic side of the inner membrane or with other inner membrane components of the TTSS (Fig. 2A). A less-abundant 60-kDa band was also detected in the inner membrane.
EscC localization is dependent on EscV and EscN.
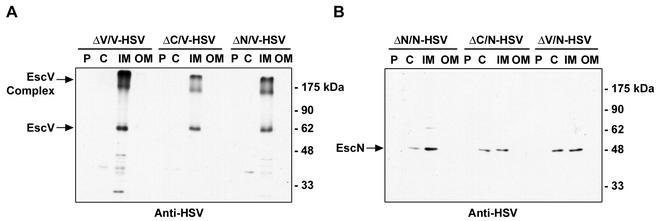
To determine whether the localization of EscC, EscV, or EscN was affected by other type III apparatus components, a series of trans-complementation experiments were performed. As seen in Fig. 3A, the localization of EscV-HSV was not affected in the escC and escN mutants. Similarly, the localization of EscN-HSV was not affected in escC and escV mutants transformed with pEscN-HSV.
FIG. 3.
Localization of EscV and EscN does not require other examined type III apparatus components. Bacteria were fractionated into periplasmic (P), cytoplasmic (C), and inner membrane (IM) and outer membrane (OM) fractions by lysozyme treatment, ultracentrifugation, and selective detergent extraction, respectively. Samples (10 μg of each fraction) were resolved by SDS-10% PAGE, transferred to a nitrocellulose filter, and probed with anti-HSV antisera. (A) Localization of EscV-HSV. (B) Localization of EscN-HSV.
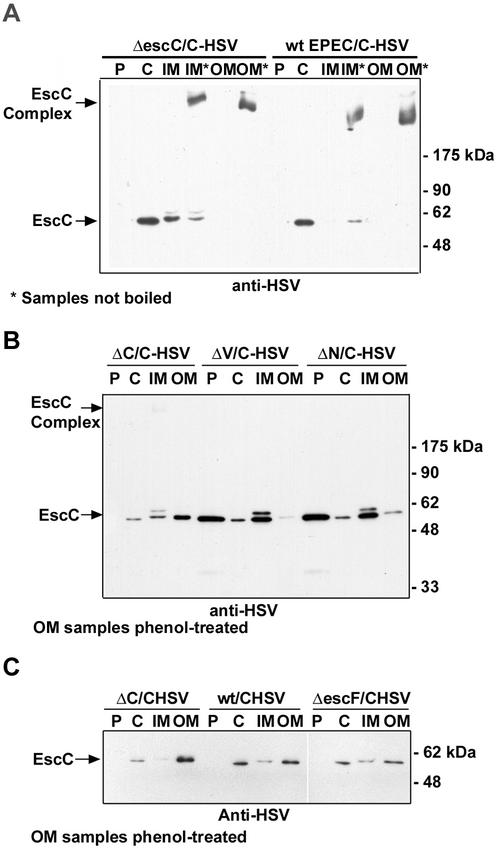
Based on its putative cleavable signal sequence, EscC is predicted to be exported across the inner membrane via the sec-dependent pathway and inserted into the outer membrane. The secretin InvG in Salmonella serovar Typhimurium has been shown to have a cleavable sec-dependent signal sequence (37). Wild-type EPEC transformed with pEscC-HSV was fractionated to ensure that any altered distribution of EscC-HSV in the escV and escN mutants was not due to competition with wild-type EscC expressed from the chromosomal copy of the gene (Fig. 4A). ΔescC/pEscC-HSV and wild-type EPEC/pEscC-HSV had the same pattern of EscC-HSV distribution: monomeric EscC was detected in the cytoplasmic and inner membrane fraction, and an EscC complex was detected in the outer membrane fraction when the samples were not boiled prior to electrophoresis. In contrast, when trans-complementation experiments were performed to examine the location of EscC-HSV in escV and escN mutants, they revealed that EscC localization was altered (Fig. 4B). A large quantity of monomeric EscC was detected in the periplasmic fractions of escV and escN mutants, compared to no detectable EscC in the periplasmic fraction of wild-type EPEC or ΔescC/pEscC-HSV. Furthermore, the monomeric EscC was also more abundant in the inner membrane fraction, but much less EscC was observed in the outer membrane fraction of the trans-complemented strains. Equivalent amounts of EscC-HSV were produced by the mutants and wild-type EPEC, suggesting that the altered distribution was not due to altered expression (data not shown). A similar pattern of EscC distribution in the mutants was also seen by using a suboptimal polyclonal antibody directed against EscC (data not shown), indicating that these results are not an artifact due to the presence of the HSV tag. We next investigated if EscC outer membrane insertion occurs before or after type III needle assembly, by examining the location of EscC-HSV in a needle component mutant (escF) (kindly provided by Akio Abe) (58). EscC-HSV was correctly localized in the escF mutant, giving the same pattern of distribution as the wild type and the ΔescC mutant (Fig. 4C). This indicates that EscC localization is independent of needle formation. Collectively, these data strongly suggest that insertion of EscC into the outer membrane requires both the TTSS and the sec-dependent secretion system.
FIG. 4.
EscC localization is dependent on EscV and EscN but not on EscF. Bacteria were fractionated into periplasmic (P), cytoplasmic (C), and inner membrane (IM) and outer membrane (OM) fractions by lysozyme treatment, ultracentrifugation, and selective detergent extraction, respectively. Samples (10 μg of each fraction) were resolved by SDS-10% PAGE, transferred to a nitrocellulose filter, and probed with anti-HSV antisera. (A) EscC-HSV distribution is the same in wild-type (wt) EPEC and the ΔescC mutant. Asterisks indicate samples that were not boiled prior to electrophoresis. (B) EscC-HSV distribution in ΔescC, ΔescV, and ΔescN mutants. The outer membrane fraction was treated with phenol prior to addition of SDS sample buffer. (C) EscC-HSV distribution in the wild type and the ΔescC and ΔescF mutants. The outer membrane fraction was treated with phenol prior to addition of SDS sample buffer.
DISCUSSION
Several protein transport systems have been characterized, and pathways have been elucidated, by creating mutations in putative apparatus components and examining where effectors accumulate. For example, in the flagellar export apparatus, hook type proteins were exported to the periplasm in rod mutants, indicating that rod protein export does not precede hook type protein export (44). In this study we examined apparatus components and effector localization by constructing nonpolar deletion mutants for the genes encoding EscN, EscV, and EscC. Bacterial fractionation revealed the same pattern of Tir and EspB localization with all three mutants: Tir and EspB accumulated in the cytoplasmic and inner membrane fractions in the mutants, whereas in wild type EPEC, only small amounts of these effectors were detected in the cytoplasm. The data suggest that fully functional type III components are needed for the correct biogenesis of the type III apparatus and for any degree of export of effectors by EPEC.
Further, this biochemically demonstrates that the type III secretion apparatus is a continuous channel. This is in agreement with the central continuous channel observed in electron microscopic images of the Salmonella serovar Typhimurium and Shigella flexneri type III secretion apparatuses (3, 33). While there are periplasmic intermediates in the export of rod and hook type proteins via the flagellar system (44) and analogous intermembrane space intermediates in the mitochondrial import system (50), no periplasmic Tir or EspB intermediates were detected in the wild-type or mutant EPEC TTSS. This suggests that type III secretion of translocator or effector type substrates is direct and that there are no intermediates. Contrary evidence has been presented for the type III system in the plant pathogen Pseudomonas syringae, where in an hrcC mutant (EscC homologue), an effector, HrpZ, was detected in the periplasmic fraction (4). This difference could be due to different fractionation methods, a difference between these two pathogens, or perhaps a difference between plant and animal pathogens.
EscV-HSV and EscN-HSV were localized by bacterial fractionation, experimentally confirming their predicted bacterial locations. trans-complementation experiments revealed that the localization of EscV and EscN in the bacteria was unaffected by mutations in the other type III components that were examined. EscV has a putative N-terminal cleavable signal sequence and multiple transmembrane domains and thus is most likely exported via the sec-dependent pathway and inserted into the inner membrane. EscN homologues are predicted to be located in the cytoplasm, where they interact with the membrane-bound components of the TTSS, thereby energizing the system (26), but EscN is the first of the type III virulence secretion system ATPases to be localized in the cytoplasm and associated with the inner membrane. The flagellar EscN homologue FliI is present in the cytoplasm and in association with the membrane in Caulobacter crescentus (60). The FliI of Salmonella serovar Typhimurium has been characterized enzymatically and proposed to have ATPase activity in the C terminus and to interact with other components of the flagellar export apparatus via its N terminus (2, 16). FliI also interacts with a number of components of the flagellar export apparatus and its substrates (45, 59), suggesting that the ATPase could directly facilitate transfer of effector proteins. Studies with Shigella and Salmonella demonstrated that mutants of EscN homologues do not have needles, suggesting that the ATPase is required for export of all nonmembrane components of the TTSS (39, 62).
In this study, EscC was found as a complex in the outer membrane of EPEC, supporting previous studies with EscC homologues in other TTSSs (5, 12, 35, 54). The EscC complex could be dissociated to the monomer only by phenol treatment, again supporting findings with other secretins (12, 22, 23, 35). It has been shown that the C-terminal β domain of PulD of K. oxytoca is responsible for multimer stability and that the N-terminal domain is responsible for multimer formation (20). It has been shown experimentally that InvG has a cleavable signal sequence at residue 25 and PulD has an export signal capable of directing the export of alkaline phosphatase, indicating that secretins are exported by the sec-dependent pathway and inserted into the outer membrane (11, 37).
Unexpectedly, the localization of EscC was altered in escV and escN mutants: large quantities of EscC were found in the periplasm. This indicates that correct insertion and function of EscC in the outer membrane require not only the general secretion pathway but also components of the type III apparatus in EPEC. This contrasts with previous indications for other secretins (32, 37). Previous data have demonstrated that small lipoproteins are required to increase the efficiency for the correct localization and functioning of InvG in Salmonella serovar Typhimurium (5, 6, 61), MxiD in Shigella (57), and YscC in Y. enterocolitica (35). In the absence of the lipoproteins PrgH and PrgK, the amount of the InvG secretin complex in Salmonella serovar Typhimurium is reduced (61). A precedent for such a requirement exists in the general secretory pathways of K. oxytoca, in which the outer membrane secretin PulD requires its chaperone PulS for insertion into the outer membrane, where they form a complex (22, 48). These small lipoproteins are called secretin pilots, although we have not found a protein that fits this description in EPEC. The results from previous studies differ from those of the present study in that in the former, all of the proteins associated with secretin insertion into the outer membrane are outer membrane or outer-membrane-associated components themselves, whereas the present study demonstrates that both a cytoplasmic and an inner membrane component of the type III apparatus are also required for insertion of the outer membrane type III secretin.
Recently, the effects of mutations in each of the type III secretion apparatus genes in Salmonella serovar Typhimurium on the assembly of the needle complex were examined (61). This work demonstrated that most mutants displayed normal base substructures on the bacterial envelope but lacked the needle structure. Notably, mutants of Salmonella serovar Typhimurium's EscV homologue, invA (39, 61), and mutants of Shigella's EscN homologue, spa47 (62), have wild-type levels of secretin in the needle complex fraction and apparently normal base substructure, although without the needle portion. While the secretin was detected in the needle complex in these studies, it remains to be shown if the protein was in the outer membrane.
EscF is a component of the TTSS needle in EPEC (7, 58, 69). Recently, less EscC was found in a needle complex fraction from an escF mutant than in that from wild-type EPEC (58). In the present study, EscC's outer membrane localization was not disturbed in an escF mutant, indicating that EscC's assembly does not depend on EscF and suggesting that EscC's outer membrane localization precedes needle formation. In agreement with this finding, both Shigella and Salmonella needle component mutants have normal amounts of their respective type III secretins in partially purified type III secretion complexes (61, 62).
Previous models of the assembly of the type III apparatus are divided into two stages (32, 61): (i) a sec-dependent phase, where the membrane-bound components are exported via the general secretory pathway and self-assemble to form the type III apparatus, followed by (ii) a sec-independent or type III-dependent phase, where there is continued assembly of the needle complex and the type III apparatus is utilized to secrete the needle components. Our study indicates that the type III apparatus is required for more than just assembly of the needle complex.
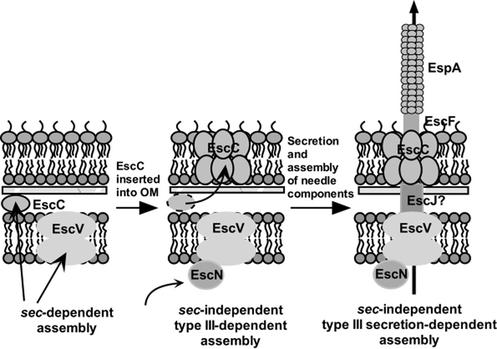
Collectively, the data from the present study suggest a three-stage model for assembly of the TTSS in EPEC (Fig. 5). The first is a sec-dependent phase where the inner membrane and membrane-spanning components are exported via the general secretion pathway and the outer membrane secretin is exported across the inner membrane to the periplasmic region, also via the sec apparatus. The second stage is a sec-independent, type III-dependent phase where EscC is inserted into the outer membrane with the aid of the ATPase EscN, the inner membrane EscV, and possibly other type III apparatus proteins. It is likely that other type III apparatus components are also involved, since there does not appear to be a direct link between EscN or EscV and EscC. It seems unlikely that EscC actually goes through any type III pore; rather, it is somehow helped into position by type III apparatus components. The third stage is a sec-independent, type III secretion-dependent phase where the type III export machinery is utilized to secrete and assemble the needle components.
FIG. 5.
Model for assembly of the type III secretion apparatus in EPEC. During the first, sec-dependent phase, the inner membrane and membrane-spanning components are exported via the general secretion pathway, and the outer membrane secretin EscC is exported across the inner membrane to the periplasm via the general secretion pathway. The second phase is sec independent and type III dependent; EscC is inserted into, and oligomerizes in, the outer membrane with the aid of the ATPase EscN, the inner membrane protein EscV, and possibly other type III apparatus proteins. Finally, in the third phase, which is sec independent and type III secretion dependent, the type III secretion apparatus machinery is utilized to secrete and assemble the needle components.
In summary, we have shown that type III secretion is a directed process with no periplasmic intermediates of the effector proteins Tir and EspB in EPEC. EscC, EscV, and EscN were localized by bacterial fractionation, confirming their predicted locations. Moreover, we have demonstrated that correct EscC localization requires other components of the type III secretion apparatus, EscV and EscN, but not the needle component EscF. However, many questions on the assembly of the type III apparatus and needle still remain unanswered. How do the type III cytoplasmic and inner membrane apparatus proteins aid the insertion and oligomerization of the outer membrane secretin? Perhaps EscV in the inner membrane and other, membrane-spanning type III components such as EscJ interact directly with EscC in order to direct it into the outer membrane. The requirement of EscN for EscC localization in the outer membrane is unexpected and suggests that energy may be required for insertion. What is the minimal functional type III apparatus? What are the interactions between the type III apparatus proteins, and how are they dependent on one another? Through complementary biochemical and microscopy techniques, elucidation of how the type III secretion and translocation apparatus assembles and functions is feasible. This work establishes the dependence of some type III apparatus components on others and suggests that there is a hierarchy to the assembly of the TTSS in EPEC.
Acknowledgments
A.G. is supported by doctoral research awards from the Medical Research Council of Canada, the Imperial Order of the Daughters of the Empire, and the Michael Smith Foundation for Health Research. J.L.P. and B.B.F. are Howard Hughes Medical Institute (HHMI) International Research Scholars. B.B.F. is a Canadian Institutes for Health Research (CIHR) Distinguished Investigator. Operating grants from HHMI, CONACyT, and DGAPA to J.L.P. and from HHMI, CIHR, and the Canadian Bacterial Disease Network to B.B.F supported this work.
We thank Akio Abe for kindly providing the escF construct and Ilan Rosenshine for providing anti-intimin and anti-Etk antisera. We are grateful to Samantha Gruenheid, Nikhil Thomas, and Nat Brown for helpful discussions and critical reading of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Aizawa, S.-I. 2000. Flagella, p. 380-389. In Encyclopedia of microbiology, 2nd ed., vol. 2. Academic Press, New York, N.Y.
- 2.Auvray, F., A. J. Ozin, L. Claret, and C. Hughes. 2002. Intrinsic membrane targeting of the flagellar export ATPase FliI: interaction with acidic phospholipids and FliH. J. Mol. Biol. 318:941-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 4.Charkowski, A. O., H. C. Huang, and A. Collmer. 1997. Altered localization of HrpZ in Pseudomonas syringae pv. syringae hrp mutants suggests that different components of the type III secretion pathway control protein translocation across the inner and outer membranes of gram-negative bacteria. J. Bacteriol. 179:3866-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crago, A. M., and V. Koronakis. 1998. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol. Microbiol. 30:47-56. [DOI] [PubMed] [Google Scholar]
- 6.Daefler, S., and M. Russel. 1998. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol. Microbiol. 28:1367-1380. [DOI] [PubMed] [Google Scholar]
- 7.Daniell, S. J., N. Takahashi, R. Wilson, D. Friedberg, I. Rosenshine, F. P. Booy, R. K. Shaw, S. Knutton, G. Frankel, and S. Aizawa. 2001. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell. Microbiol. 3:865-871. [DOI] [PubMed] [Google Scholar]
- 8.de Grado, M., A. Abe, A. Gauthier, O. Steele-Mortimer, R. DeVinney, and B. B. Finlay. 1999. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell. Microbiol. 1:7-17. [DOI] [PubMed] [Google Scholar]
- 9.de Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 11.d'Enfert, C., I. Reyss, C. Wandersman, and A. P. Pugsley. 1989. Protein secretion by Gram-negative bacteria. J. Biol. Chem. 264:17462-17468. [PubMed] [Google Scholar]
- 12.Deng, W. L., and H. C. Huang. 1999. Cellular locations of Pseudomonas syringae pv. syringae HrcC and HrcJ proteins, required for harpin secretion via the type III pathway. J. Bacteriol. 181:2298-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Kramer, C. Deibel, C. A. Guzman, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 15.Eichelberg, K., C. C. Ginocchio, and J. E. Galan. 1994. Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J. Bacteriol. 176:4501-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan, F., and R. M. Macnab. 1996. Enzymatic characterization of FliI. An ATPase involved in flagellar assembly in Salmonella typhimurium. J. Biol. Chem. 271:31981-31988. [DOI] [PubMed] [Google Scholar]
- 17.Filip, C., G. Fletcher, J. L. Wulff, and C. F. Earhart. 1973. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier, A., M. de Grado, and B. B. Finlay. 2000. Mechanical fractionation reveals structural requirements for enteropathogenic Escherichia coli Tir insertion into host membranes. Infect. Immun. 68:4344-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genin, S., and C. A. Boucher. 1994. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol. Gen. Genet. 243:112-118. [DOI] [PubMed] [Google Scholar]
- 20.Guilvout, I., K. R. Hardie, N. Sauvonnet, and A. P. Pugsley. 1999. Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J. Bacteriol. 181:7212-7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock, R. E., and H. Nikaido. 1978. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J. Bacteriol. 136:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie, K. R., S. Lory, and A. P. Pugsley. 1996. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 15:978-988. [PMC free article] [PubMed] [Google Scholar]
- 23.Hardie, K. R., A. Seydel, I. Guilvout, and A. P. Pugsley. 1996. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol. Microbiol. 22:967-976. [DOI] [PubMed] [Google Scholar]
- 24.Hartland, E. L., S. J. Daniell, R. M. Delahay, B. C. Neves, T. Wallis, R. K. Shaw, C. Hale, S. Knutton, and G. Frankel. 2000. The type III protein translocation system of enteropathogenic Escherichia coli involves EspA-EspB protein interactions. Mol. Microbiol. 35:1483-1492. [DOI] [PubMed] [Google Scholar]
- 25.Hirano, T., T. Minamino, and R. M. Macnab. 2001. The role in flagellar rod assembly of the N-terminal domain of Salmonella FlgJ, a flagellum-specific muramidase. J. Mol. Biol. 312:359-369. [DOI] [PubMed] [Google Scholar]
- 26.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 28.Ilan, O., Y. Bloch, G. Frankel, H. Ullrich, K. Geider, and I. Rosenshine. 1999. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 18:3241-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellermann, O., and S. Szmelcman. 1974. Active transport of maltose in Escherichia coli K12: involvement of “periplasmic” maltose binding protein. Eur. J. Biochem. 47:139-149. [DOI] [PubMed] [Google Scholar]
- 31.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 32.Kimbrough, T. G., and S. I. Miller. 2002. Assembly of the type III secretion needle complex of Salmonella typhimurium. Microbes Infect. 4:75-82. [DOI] [PubMed] [Google Scholar]
- 33.Kimbrough, T. G., and S. I. Miller. 2000. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA 97:11008-11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koster, M., W. Bitter, H. de Cock, A. Allaoui, G. R. Cornelis, and J. Tommassen. 1997. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26:789-797. [DOI] [PubMed] [Google Scholar]
- 36.Kresse, A. U., M. Rohde, and C. A. Guzman. 1999. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect. Immun. 67:4834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 38.Kubori, T., N. Shimamoto, K. Yamaguchi, K. Namba, and S.-I. Aizawa. 1992. Morphological pathway of flagellar assembly in Salmonella typhimurium. J. Mol. Biol. 226:433-446. [DOI] [PubMed] [Google Scholar]
- 39.Kubori, T., A. Sukhan, S. I. Aizawa, and J. E. Galan. 2000. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl. Acad. Sci. USA 97:10225-10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liberek, K., C. Georgopoulos, and M. Zylicz. 1988. Role of the Escherichia coli DnaK and DnaJ heat shock proteins in the initiation of bacteriophage lambda DNA replication. Proc. Natl. Acad. Sci. USA 85:6632-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macnab, R. M. 1999. The bacterial flagellum: reversible rotary propellor and type III export apparatus. J. Bacteriol. 181:7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin, P. R., M. Hobbs, P. D. Free, Y. Jeske, and J. S. Mattick. 1993. Characterization of pilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 9:857-868. [DOI] [PubMed] [Google Scholar]
- 43.Michiels, T., J.-C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. R. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173:4994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minamino, T., and R. M. Macnab. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 181:1388-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minamino, T., and R. M. Macnab. 2000. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 35:1052-1064. [DOI] [PubMed] [Google Scholar]
- 46.Nataro, J., and J. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikaido, H. 1994. Isolation of outer membranes. Methods Enzymol. 235:225-235. [DOI] [PubMed] [Google Scholar]
- 48.Nouwen, N., N. Ranson, H. Saibil, B. Wolpensinger, A. Engel, A. Ghazi, and A. P. Pugsley. 1999. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc. Natl. Acad. Sci. USA 96:8173-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nouwen, N., H. Stahlberg, A. P. Pugsley, and A. Engel. 2000. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J. 19:2229-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfanner, N., and A. Geissler. 2001. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell. Biol. 2:339-349. [DOI] [PubMed] [Google Scholar]
- 51.Plano, G. V., S. S. Barve, and S. C. Straley. 1991. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J. Bacteriol. 173:7293-7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 53.Plano, G. V., and S. C. Straley. 1993. Multiple effects of lcrD mutations in Yersinia pestis. J. Bacteriol. 175:3536-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plano, G. V., and S. C. Straley. 1995. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J. Bacteriol. 177:3843-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandkvist, M. 2001. Type II secretion and pathogenesis. Infect. Immun. 69:3523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuch, R., and A. T. Maurelli. 2001. MxiM and MxiJ, base elements of the Mxi-Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J. Bacteriol. 183:6991-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekiya, K., M. Ohishi, T. Ogino, K. Tamano, C. Sasakawa, and A. Abe. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98:11638-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva-Herzog, E., and G. Dreyfus. 1999. Interaction of FliI, a component of the flagellar export apparatus, with flagellin and hook protein. Biochim. Biophys. Acta 1431:374-383. [DOI] [PubMed] [Google Scholar]
- 60.Stephens, C., C. Mohr, C. Boyd, J. Maddock, J. Gober, and L. Shapiro. 1997. Identification of the fliI and fliJ components of the Caulobacter flagellar type III protein secretion system. J. Bacteriol. 179:5355-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galan. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor, K. A., C. B. O'Connell, P. W. Luther, and M. S. Donnenberg. 1998. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect. Immun. 66:5501-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thanassi, D. G., and S. J. Hultgren. 2000. Multiple pathways allow protein secretion across the bacterial outer membrane. Curr. Opin. Cell Biol. 12:420-430. [DOI] [PubMed] [Google Scholar]
- 65.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vidal, S. M., E. Pinner, P. Lepage, S. Gauthier, and P. Gros. 1996. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J. Immunol. 157:3559-3568. [PubMed] [Google Scholar]
- 67.Wachter, C., C. Beinke, M. Mattes, and M. A. Schmidt. 1999. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 31:1695-1707. [DOI] [PubMed] [Google Scholar]
- 68.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson, R. K., R. K. Shaw, S. Daniell, S. Knutton, and G. Frankel. 2001. Role of EscF, a putative needle complex protein, in the type III protein translocation system of enteropathogenic Escherichia coli. Cell. Microbiol. 3:753-762. [DOI] [PubMed] [Google Scholar]
- 70.Woestyn, S., A. Allaoui, P. Wattiau, and G. R. Cornelis. 1994. YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol. 176:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff, C., I. Nisan, E. Hanski, G. Frankel, and I. Rosenshine. 1998. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143-155. [DOI] [PubMed] [Google Scholar]