Short abstract
Semaphorins are secreted, transmembrane, and GPI-linked proteins found in mammals, Drosophila, nematodes, and crustaceans. They alter the cytoskeleton and the organization of actin filaments and the microtubule network, and are important in a wide range of functions, including neural connectivity, angiogenesis, immunoregulation, and cancer.
Abstract
Semaphorins are secreted, transmembrane, and GPI-linked proteins, defined by cysteine-rich semaphorin protein domains, that have important roles in a variety of tissues. Humans have 20 semaphorins, Drosophila has five, and two are known from DNA viruses; semaphorins are also found in nematodes and crustaceans but not in non-animals. They are grouped into eight classes on the basis of phylogenetic tree analyses and the presence of additional protein motifs. The expression of semaphorins has been described most fully in the nervous system, but they are also present in most, or perhaps all, other tissues. Functionally, semaphorins were initially characterized for their importance in the development of the nervous system and in axonal guidance. More recently, they have been found to be important for the formation and functioning of the cardiovascular, endocrine, gastrointestinal, hepatic, immune, musculoskeletal, renal, reproductive, and respiratory systems. A common theme in the mechanisms of semaphorin function is that they alter the cytoskeleton and the organization of actin filaments and the microtubule network. These effects occur primarily through binding of semaphorins to their receptors, although transmembrane semaphorins also serve as receptors themselves. The best characterized receptors for mediating semaphorin signaling are members of the neuropilin and plexin families of transmembrane proteins. Plexins, in particular, are thought to control many of the functional effects of semaphorins; the molecular mechanisms of semaphorin signaling are still poorly understood, however. Given the importance of semaphorins in a wide range of functions, including neural connectivity, angiogenesis, immunoregulation, and cancer, much remains to be learned about these proteins and their roles in pathology and human disease.
Gene organization and evolutionary history
Semaphorins are a large and diverse family of widely expressed secreted and membrane-associated proteins, which are conserved both structurally and functionally across divergent animal phyla. This diversity in expression, structure, and function is highlighted in the manner in which a number of the semaphorins were originally characterized. The first semaphorin to be discovered, the grasshopper transmembrane protein semaphorin-1a (Sema-1a; originally named Fasciclin IV), was identified in a screen for molecules with distinctive temporal and spatial distributions in the developing grasshopper nervous system [1]. In parallel experiments, a neuronal growth cone collapsing factor associated with chicken brain membranes was biochemically purified and found to be a secreted semaphorin (Sema3A; originally named Collapsin) [2]. Separate experimentation and molecular characterization revealed that an antigen first observed in the 1970s as present in high frequency on human red blood cells, the John Milton Hagen (JMH) human blood group antigen, was a glycosylphosphatidylinositol (GPI)-linked semaphorin (Sema7A; also known as CDw108) [3,4]. And work in the human immune system showed that an antigen first characterized in 1992 for its presence on the surface of T lymphocytes was a transmembrane semaphorin (Sema4D; originally named CD100) [5].
Sequences encoding a number of different semaphorins have since been identified in nematode worms, insects, crustaceans, vertebrates, and viruses, but to date they have not been described in protozoans, plants, or the most primitive metazoans. Although initially given various and often conflicting names, these sequences have now been consolidated into one family called the semaphorins; the name is derived from the word 'semaphore', meaning to convey information by a signaling system [6,7]. The semaphorin gene family currently includes 20 members in mice and humans and five in Drosophila, and they can be divided into eight classes, 1-7 and V (Figures 1, 2) [7]. Vertebrates have members in classes 3-7, whereas classes 1 and 2 are known only in invertebrates and class V only in viruses.
Figure 1.
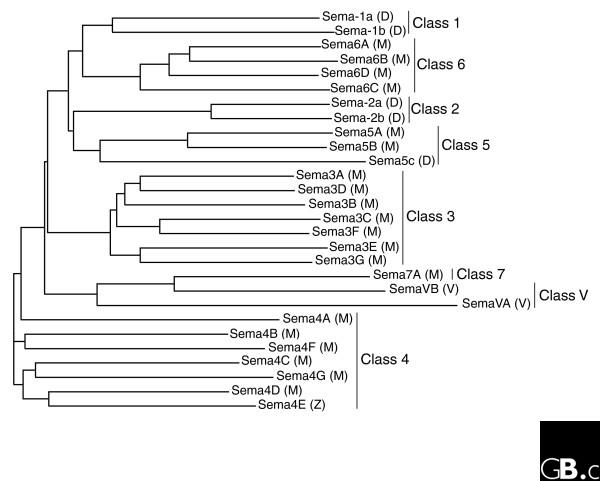
A phylogenetic tree of semaphorin sequences, showing groupings of related semaphorin genes and their organization into different classes. D, Drosophila; M, mouse; V, viral; Z, sequence identified only in zebrafish and not in mammals. A Sema5D has also been described, but our analysis indicates that it is a splice variant of Sema5B. Protein sequences were aligned using ClustalW in Vector NTI software and the tree was generated using the neighbor-joining method, ignoring positions with gaps.
Figure 2.
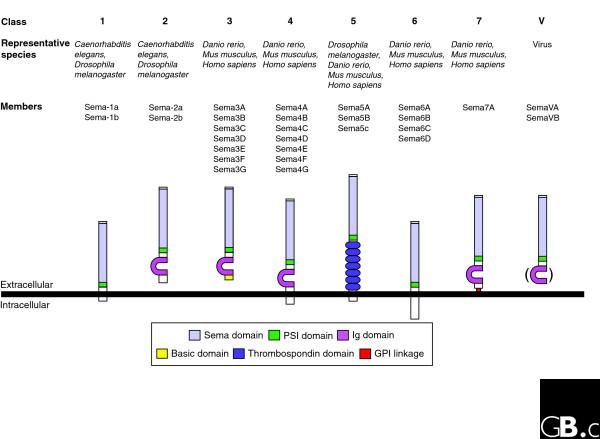
Primary structures of members of the semaphorin family. All proteins are shown with their amino termini to the top. Class 1 semaphorins are invertebrate transmembrane proteins and are structurally very similar to the class 6 semaphorins of vertebrates. Class 2 semaphorins (also from invertebrates) are secreted; they are structurally similar to vertebrate class 3 semaphorins, which have a stretch of highly basic amino acids in their carboxy-terminal region. Class 4, 6, and 7 semaphorins have been identified only in vertebrates. Class 4-6 semaphorins are transmembrane proteins. Class 5 semaphorins are present in both vertebrates (Sema5A, Sema5B) and invertebrates (Sema5c) and contain seven canonical type 1 thrombospondin repeats (TSRs). Class 6 semaphorins contain variable, alternatively spliced cytoplasmic portions. The lone class 7 sema (Sema7A) contains a membrane-associated GPI moiety at its carboxy terminus. Class V semaphorins are highly similar to class 7 semaphorins and are found in DNA viruses, including vaccinia (a close relative to the cowpox virus), human smallpox (variola virus), fowlpox, mousepox (ectromelia virus), and alcelaphine herpesvirus type 1 virus (AHV). Some class V semaphorins (the SemaVA proteins) do not contain an Ig domain, whereas others do (SemaVB proteins). Sema, semaphorin; PSI, plexin-semaphorin-integrin; Ig, immunoglobulin-like; GPI, glycosylphosphatidylinositol.
Semaphorin genes are dispersed throughout the genome, typically including several exons per gene, and are known to be alternatively spliced. There is considerable sequence diversity within the family: with a few exceptions, individual members are not more than about 50% identical to each other at the amino-acid level (see Additional data file 1).
Characteristic structural features
The eight main classes of semaphorins [7] differ in sequence and overall structural characteristics, but all members of the family contain a conserved extracellular domain of about 500 amino acids termed the semaphorin (sema) domain (Figure 2). This domain shows considerably higher conservation among the different semaphorins and across phyla than do the full-length proteins (see Additional data file 2). In addition to several blocks of conserved amino acids, the sema domain is characterized by highly conserved cysteine residues that have been found to form intrasubunit disulfide bonds [8]. Crystal structures have revealed that the sema domain of both the mouse secreted semaphorin Sema3A and the human transmembrane semaphorin Sema4D fold in a variation of the β propeller topology, a common topology that occurs in proteins with diverse functions (reviewed in [8]). Interestingly, these sema domains fold in a manner that is most similar to the β propeller topology of integrins and low-density lipoprotein (LDL) receptors.
The sema domain is also a critical component through which semaphorins mediate their effects [9-11]. In particular, an approximately 70-amino-acid region within the sema domain is important for the effects of Sema3A on repulsive axon guidance and the collapse of the growing tip or growth cones of axons, which stops their extension [9]. Structurally, this portion of the sema domain of Sema3A and Sema4D appears to correspond to blade three of the seven-bladed β propeller topology [8]. Interestingly, a small stretch of amino acids homologous to tarantula hanatoxin, a K+ and Ca2+ ion-channel blocker, is also important for the growth-cone-collapsing effects of Sema3A [12].
Immediately to the carboxy-terminal side of the sema domain, semaphorins contain a plexin-semaphorin-integrin (PSI) domain (Figure 2). This small stretch of cysteine-rich residues has also been referred to as a MET-related sequence (MRS) or a cysteine-rich domain (CRD). With the exception of some viral semaphorins, all examples of proteins containing a sema domain have a PSI domain [8]. Crystal-structure analysis indicates that this domain is highly conserved, but its three-dimensional position relative to the sema domain can vary among semaphorins [8]. Semaphorins also have consensus N-linked glycosylation sites and may be alternatively spliced (as in Drosophila Sema-1a [13], and mammalian Sema3F [14] and Sema6A [15]), although little is known about the significance of these modifications.
In contrast to these defining characteristics, individual semaphorins have a number of distinguishing features. Semaphorins vary in their membrane anchorage, and include secreted, transmembrane, and GPI-linked family members (Figure 2). They may also contain additional sequence motifs, including a single C2-class immunoglobulin-like (Ig) domain, a stretch of highly basic amino acids, and/or seven canonical type 1 thrombospondin repeats (TSRs; Figure 2). These additional domains are responsible for at least some of their functional effects; for example, the Ig domain and basic tail of chicken Sema3A potentiate the effect of its sema domain in growth-cone collapse [9], and the thrombospondin repeats of mammalian Sema5A are important in regulating the effect of Sema5A on axon guidance [11,16].
Localization and function
As a group, semaphorins are expressed in most tissues and this expression varies considerably with age. The expression patterns of the individual semaphorins are best characterized in the nervous system, particularly during development, where most, or perhaps all, semaphorins are widely expressed in the nervous system by neuronal and non-neuronal cells (reviewed in [17]; see Table 1 for details of the expression and functions of all members of the family and associated references). Semaphorins are also widely expressed in many organ systems and their derivatives, including the cardiovascular, endocrine, gastrointestinal, hepatic, immune, musculoskeletal, renal, reproductive, and respiratory systems.
Table 1.
Expression and function of semaphorins
| Semaphorin | Species | Expression (with representative references) | Functions (with representative references) |
| Sema-1a | Insects and worms | Epidermis [1], neurons [1,6,13,50] | Cell migration [110], digestion/defecation [110], fecundity [110], morphogenesis [110], neural connectivity [1,13] |
| Sema-1b | Insects and worms | Glia [55], oocytes [55] | Cell migration [110], morphogenesis [110], neural connectivity [110] |
| Sema-2a | Insects and worms | Epidermis [6], epithelium [6], gonads [6], muscles [6], neurons [6] | Cell migration [111], morphogenesis [111], neural connectivity [112] |
| Sema-2b | Insects | Unknown | Unknown |
| Sema3A | Vertebrates | Adipose tissue [56,57], bone [58], cartilage [58], cancer cells [59], connective tissue [60], endothelial cells [61], epithelium [62], glia [25], gut [62], heart [2,58], kidney [63], limb [58], lung [2], meningeal cells [64], muscle [2,57], neurons [2,58], pituitary [62], placenta [65], scar tissue [66], teeth [67], umbilical cord [65], uterus [65] | Bone formation [113], cancer-cell chemotaxis [114], cartilage formation [113], cell death [115], cell adhesion and aggregation [61,116], cell migration and patterning [117-119], cell proliferation [120], cytoskeletal organization [2], heart formation [113], lung formation [121], neural connectivity [2,113,122], vasculogenesis [61,123] |
| Sema3B | Vertebrates | Cancer cells [68], endothelial cells [61], glia [69], mammary gland [70], muscle [60], neurons [60], teeth [71] | Cell death [124], cytoskeletal organization [125], neural connectivity [126], tumor suppression [124] |
| Sema3C | Vertebrates | Cancer cells [59], connective tissue [60], endothelial cells [71], fibroblasts [53], glia [72], lung [60,73], macrophages [53], mammary gland [70], neurons [60], skeleton [60], teeth [71] | Cardiovascular development [127], cell survival [128], cytoskeletal organization [9], heart formation [127], lung formation [73], neural connectivity [9,128] |
| Sema3D | Vertebrates | Bone [74], cartilage [75], endothelial cells [61], epidermis [74], fibroblasts [76], glia [72], heart [77], meninges [74], muscle [74], neurons [74] | Neural connectivity [75] |
| Sema3E | Vertebrates | Cancer cells [78], ear [79], endothelial cells [61], lung [78], nervous tissue [25,74,80], skeleton [78], teeth [71] | Cell growth [33], cell migration [33], cytoskeletal organization [80], neural connectivity [80,129], tumor metastasis [33], vascular patterning [130] |
| Sema3F | Vertebrates | Cancer cells [81], dermis [82], ependyma [82], epithelium [82], eye [82], gonads [81], gut [81], heart [81], kidney [81], lung [81,82], muscle [81], neurons [82], pancreas [81], prostate [81], skin [82], spleen [81], submandibular gland [82], teeth [67], thymus [81], thyroid gland [82] | Angiogenesis [131], cell attachment [132], cell migration [133,134], cell proliferation [133], cytoskeletal organization [14,135], lung formation [73], neural connectivity [82,136], tumor metastasis [137], tumor suppression [138], synaptic transmission [20] |
| Sema3G | Vertebrates | Heart [83], kidney [83], lung [83], meninges [83], neurons [83], placenta [83] | Cell migration [134], neural connectivity [83] |
| Sema4A | Vertebrates | Epithelial cells [19], glia [25], immune cells [84,85], mammary gland [70], neurons [60], teeth [71] | Cell survival [19], cytoskeletal organization [139], lymphocyte activation and immune responses [84,85], neural connectivity [139], retina and visual system [19] |
| Sema4B | Vertebrates | Glia [25], immune cells [86], neurons [60,87], teeth [71] | Unknown |
| Sema4C | Vertebrates | Bone [76], ear [88], glia [25], immune cells [86], kidney [88], lung [88], muscle [89], neurons [88,90], regenerating muscle [89], teeth [88], pituitary [88] | Myogenesis [89] |
| Sema4D | Vertebrates | Glia [24], gonads [91], gut [91], immune cells [86,91], kidney [91], heart [91], lung [91], lymph node [91], mammary gland [70], muscle [91], neurons [92], placenta [91], prostate [91], spleen [91], teeth [71], thymus [91] | Angiogenesis [140,141], cell aggregation and adhesion [91,142], cell death [143], cell differentiation [91], cell migration [35,140,141], cell proliferation [144], cell survival [91,145], cytoskeletal organization [143,146], invasive/cancerous growth [147], immune responses [91,144], neural connectivity [24,145,146] |
| Sema4E | Zebrafish | Epithelium [93], nervous system [93] | Neural connectivity [148] |
| Sema4F | Vertebrates | Glia [72], immune cells [86], lung [94], mammary gland [70], neurons [94,95], teeth [71] | Cytoskeletal organization [94], neural connectivity [94] |
| Sema4G | Vertebrates | Ear [96], epithelium [96], glia [72], gut [96], hair follicles [96], kidney [96], liver [96], neurons [96], pituitary [96], teeth [71] | Unknown |
| Sema5A | Vertebrates | Cancer cells [97], glia [25], heart [98], kidney [98], liver [98], lung [98], muscle [98], neurons [98], spleen [98], teeth [71] | Cell morphology [149], cytoskeletal organization [11], neural connectivity [11,16,25], vasculature patterning [150] |
| Sema5B | Vertebrates | Bone [76], cancer cells [97], glia [25], neurons [98], teeth [71] | Unknown |
| Sema5c | Insects | Cardiac cells [55], epidermis [97,99], gut [99], muscle [55,99], oocytes [55] | Tumor metastasis [97], tumor suppression [97] |
| Sema6A | Vertebrates | Cancer cells [100], bone [101], glia [25], gut [40], immune cells [86], kidney [40], lung [101], muscle [101], neurons [101], meninges [101], teeth [71] | Angiogenesis [100], cell migration [100], cytoskeletal organization [40], neural connectivity [40,49,151] |
| Sema6B | Vertebrates | Bone [76], cancer cells [102], glia [72], heart [39], liver [39], lung [39], muscle [39], nervous tissue [39,103], teeth [71] | Neural connectivity [151] |
| Sema6C | Vertebrates | Bone [76], dermis [104], glia [72], heart [105], kidney [105], liver [105], muscle [104], neurons [104], placenta [105], teeth [71] | Cytoskeletal organization [104,105], neural connectivity [104,105] |
| Sema6D | Vertebrates | Gut [105], heart [105], kidney [105], liver [105], lung [105], muscle [105], neurons [105], placenta [105], uterus [106] | Cell migration [48], cytoskeletal organization [105], heart formation [48,152], morphogenesis [152], neural connectivity [105] |
| Sema7A | Vertebrates | Adrenal gland [107], bone [108], cancer cells [109], erythrocytes [109], fibroblasts [76], glia [72], gonads [107], gut [107], heart [107], kidney [107], lung [107], lymph nodes [107], immune cells [109], muscle [107], neurons [107], placenta [107], spleen [107], teeth [71], thymus [107] | Cell fusion [108], cell migration [108,153], immune responses [153], stimulating cytokine production [153], neural connectivity [154] |
| SemaVA | DNA viruses | Not applicable | Cell adhesion [31], cell migration [31], cell retraction [31], cytoskeletal organization [31], immunomodulation [155], proinflammatory responses [155], inducing cytokine production [156], regulating phagocytosis [157] |
| SemaVB | DNA viruses | Not applicable | Cell aggregation [156] |
No particular pattern of expression appears to define each of the different classes of semaphorins, but many are dynamically expressed in particular areas during development, and this expression often decreases with maturity. In the nervous system, for example, semaphorin expression is often associated with growing axons as they form axonal tracts, but this expression often decreases following the formation of the tracts. Interestingly, changes in the adult expression levels of semaphorins have been described following injury in neuronal and non-neuronal tissues, during tumorigenesis, and in association with other pathological conditions.
The diverse expression patterns of the different semaphorins suggest that they are important in a variety of functions during development and into adulthood. Indeed, genetic analyses in both invertebrates and vertebrates indicate that semaphorins are often required for viability and reveal, in combination with additional functional assays, distinct roles in various physiological and pathological processes in most or perhaps all tissues. These studies reveal that semaphorins on cellular processes such as adhesion, aggregation, fusion, migration, patterning, process formation, proliferation, viability, and cytoskeletal organization.
Semaphorins are best known for their roles in nervous system development, and a number of approaches in vivo and in vitro indicate that semaphorins can enable axons to find and connect with one another and their other targets (reviewed in [18]). An important way in which semaphorins guide these growing axons is by repelling them or preventing them from entering certain regions. For example, characterization of their normal expression patterns, the defects observed in particular semaphorin mutants, and assays in vivo and in vitro have revealed that at least some semaphorins form molecular boundaries to prevent axons and cells from entering inappropriate areas. Semaphorins also have roles in physiological and pathological processes in the adult. In the nervous system, altered semaphorin function has been linked to epilepsy, retinal degeneration, Alzheimer's disease, motor neuron degeneration, schizophrenia, and Parkinson's disease [19-22].
Semaphorins may also limit the ability of axons to regrow after injury and prevent abnormal sprouting of axons involved in pain or autonomic function [23-26]. In the immune system, semaphorins are critical for various phases of the immune response (Table 2; reviewed in [27]). Semaphorins are also involved in cancer progression, by affecting chemotaxis, viability, tumorigenesis, metastasis, and angiogenesis (reviewed in [28]). More recently, semaphorins have also been implicated in vascular health and heart disease (reviewed in [29]).
Table 2.
Receptors and signaling proteins associated with semaphorins
| Semaphorin | Binding receptors (with representative references) | Signaling proteins (with representative references) | 'Reverse' signaling (with representative references) |
| Sema-1a | PlexA [158,159] | OTK [168], Gyc76c [169], MICAL [170], Nervy [171], PKA [171], Rac [172] | ena [50] |
| Sema-1b | PlexA [158] | - | - |
| Sema-2a | - | - | - |
| Sema-2b | - | - | - |
| Sema3A | Npn-1 [160,161], proteoglycans [162] | PlexA1, A2, A3, A4 [165,173,174], PlexD1 [175], VEGF receptor [176], L1CAM [177], integrins [61], α2-chimaerin [178], Cdc42 [179], Cdk5 [180], cGKI/PKG [181,182], Calcium channels [12], cofilin [183], CRAM [184], CRMP [185], FARP2 [45], Fes [184], Fyn [180], Go/Gi [185], guanylate cyclase [186], GSK-3 [187], LIM kinase [183], 12/15-lipoxygenase [188], MAP kinases [176], MLCK [189], nNOS [190], PI 3-kinase [181], PIPKIγ661 [45], PKA [181], PTEN [191], Rac [192], Rap1 [193], Rho [194], Rnd [195], ROCK [181], R-Ras [45] | - |
| Sema3B | Npn-1 [125], Npn-2 [125] | NrCAM [126], FAK [126], MAP kinases [126], Src [126] | - |
| Sema3C | Npn-1 [163], Npn-2 [163] | PlexD1 [196], MLCK [189], ROCK [189] | - |
| Sema3D | Npn-1 [164] | - | - |
| Sema3E | Npn-1 [164], PlexD1 [130] | Ca2+ channels [129], MAP kinases [129], PKC [129], Ras [129] | - |
| Sema3F | Npn-2 [163], Npn-1 [163] | PlexA3, A4 [173,174], NrCAM [127], E-cadherin [197], Beta-catenin [197], PI 3-kinase [198], MAP kinases [198] | - |
| Sema3G | Npn-2 [83] | - | - |
| Sema4A | Tim-2 [84] | ROCK [139] | - |
| Sema4B | - | - | PSD-95 [87] |
| Sema4C | - | - | PSD-95 [90], GIPC [207], norbin [208] |
| Sema4D | PlexB1 [165], PlexB2 [166], CD72 [167] | Met [147], Ron [199], ErbB2 [200], PlexC1 [34], integrin [201], AKT [141], Gab1 [147], LARG [146], 12/15-lipoxygenase [201], p190RhoGAP [202], PDZ-RhoGEF [146], PI 3-kinase [141], Pyk2 [141], Ras [46,203], Rho [204], Rnd [205], Src [141], MAP kinases [203], Raf [203] | CD45 [142], serine kinase [209] |
| Sema4E | - | - | - |
| Sema4F | - | - | PSD-95 [95] |
| Sema5A | PlexB3 [149], HSPG [16], CSPG [16], Syn-3 [16] | Met [149] | - |
| Sema5B | - | - | - |
| Sema5C | - | - | - |
| Sema6A | PlexA4 [151] | - | EVL [15] |
| Sema6B | PlexA4 [151] | - | Src [39] |
| Sema6C | - | - | - |
| Sema6D | PlexA1 [152] | OTK [152], VEGF receptor 2 [152] | Abl [46] |
| Sema7A | PlexC1 [165] | Integrins [154], Arg [206], FAK [154], MAP kinases [154] | Kinase activity [4] |
| SemaVA | PlexC1 [156] | Integrins [31], cofilin [31], FAK [31] | - |
| SemaVB | PlexC1 [156] | - | - |
A hyphen indicates not known. Abbreviations: Abl, Abelson tyrosine kinase; AKT, AKT serine/threonine kinase; Arg, Abl-related tyrosine kinase; CAM, cell adhesion molecule; CD45, CD45 phosphatase; Cdk5, cyclin-dependent kinase 5; CRAM, CRMP-associated molecule; CRMP, collapsing response mediator protein; cGKI, cGMP dependent protein kinase I; CSPG, chondroitin sulfate proteoglycan; ErbB2, receptor tyrosine kinase; ena, enabled; EVL, ena/VASP-like protein; FAK, focal adhesion tyrosine kinase; FARP2, FERM domain-containing GEF; Fes, feline sarcoma tyrosine kinase; Fyn, Fyn tyrosine kinase; Gab1, GRB2 associated binding protein 1; GIPC, GAIP interacting protein carboxy terminus; GSK-3, glycogen synthase kinase-3; Gyc76c, receptor guanylate cyclase 76c; HSPG, heparin sulfate proteoglycan; LARG, leukemia-associated RhoGEF; Met, receptor tyrosine kinase; MICAL, molecule interacting with CasL; MLCK, myosin light chain kinase; nNOS, neuronal nitric oxide synthase; Npn, neuropilin; OTK, off-track receptor tyrosine kinase; PI 3-kinase, phosphatidylinositol 3-kinase; PIPKIγ661, PIP kinase type I; PKA, protein kinase A; PKC, protein kinase C; PKG, protein kinase G; Plex, plexin; Pyk2, Pyk2 tyrosine kinase; PSD-95, post-synaptic density protein; PTEN, PTEN phosphatase; ROCK, Rho-associated kinase; Ron, receptor tyrosine kinase; Src, Src tyrosine kinase; Syn-3, syndecan-3; Tim, T-cell immunoglobulin domain and mucin domain; VEGF, vascular endothelial growth factor.
Mechanism
The molecular mechanisms by which semaphorins mediate their functional effects are far from clear. Semaphorin-mediated axon repulsion is a result of the modification of the axonal cytoskeleton at the growing tips or growth cones of axons. The control of axon outgrowth or growth-cone motility depends critically upon the dynamics of F-actin polymerization and depolymerization, coupled with the regulation of F-actin translocation and microtubule dynamics. Following exposure to secreted Sema3A, growth cones undergo a rapid collapse that is accompanied by the depolymerization of F-actin, a decreased ability to polymerize new F-actin, attenuated microtubule dynamics, and collapsed microtubule arrays (reviewed in [30]). The molecular mechanisms underlying these phenomena are poorly understood but may also be responsible for many of the functional effects that semaphorins have in non-neuronal tissues. For example, the cytoskeleton is required for cells to move, polarize, change shape, engulf particles, and interact with other cells; even the most divergent family member, the viral semaphorin SemaVA, induces actin cytoskeletal rearrangement in dendritic cells of the immune system and alters the ability of these cells to adhere and migrate [31].
Post-translational processing underlies at least some of the functional effects of semaphorins. Several secreted and transmembrane semaphorins undergo proteolytic processing, and this is important in semaphorin-mediated repulsive axon guidance, growth-cone collapse, cell migration, invasive growth, and metastasis (for example, see [32-35]). For example, mouse Sema3A, Sema3B, and Sema3C are synthesized as inactive precursors and become repulsive for axons upon proteolytic cleavage [32].
Oligomerization is another modification that is important for semaphorin function. The secreted vertebrate semaphorin Sema3A is a dimer [9,36,37], and dimerization is important for its activity in repulsive axon guidance and growth-cone collapse [36,37]. Cysteine residues in the carboxy terminus are important for this dimerization, although weak dimerization also occurs between sema domains [8]. Transmembrane semaphorins also form disulfide-linked dimers and depend on oligomerization for at least some of their functional effects [5,11,16,36,38-40].
Semaphorin receptors and signaling
Semaphorins exert the majority of their effects by serving as ligands and binding to other proteins through their extracellular domains. All classes of semaphorins except class 2 have been found to bind directly to members of the plexin (Plex) family of transmembrane receptors (reviewed in [41]; see Table 2 for a summary of the receptors and signaling proteins associated with semaphorins and Figure 3 for the primary structure of known semaphorin receptors). Interestingly, plexins also contain sema domains, albeit highly divergent, that are important for binding to semaphorins [8]. Several other proteins have also been identified that bind to the extracellular portions of semaphorins (Figure 3). In particular, members of the neuropilin (Npn) family of transmembrane proteins are receptors for class 3 semaphorins [30]. Both the basic tail and the sema domain of Sema3A are important for binding to Npn-1, although binding to the sema domain is weaker. Neuropilins, however, only have short cytoplasmic tails that are not required for the effects of semaphorins on axon guidance [30]. Interestingly, neuropilins also bind plexins, such that class 3 semaphorins, which bind to neuropilins, signal their effects through the cytoplasmic domain of plexins.
Figure 3.
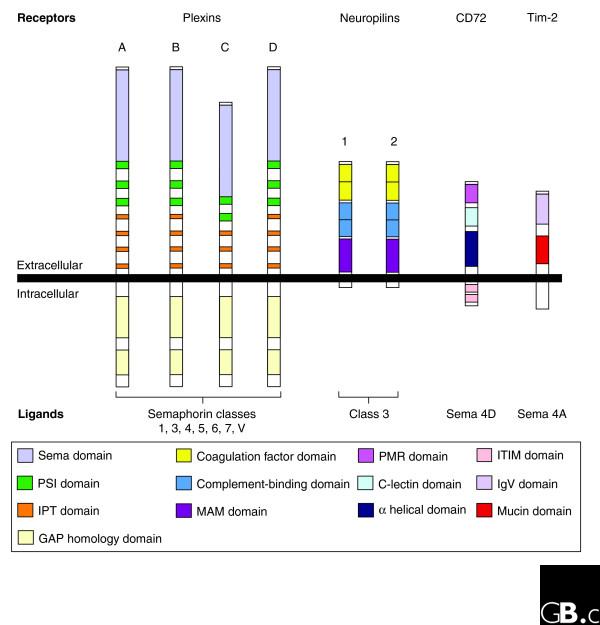
Semaphorin receptors. Members of the plexin protein family are organized into four classes (A, B, C, and D); plexins are known to bind to semaphorins from all classes except class 2, whose receptors are unknown. Class 3 semaphorins bind both members of the neuropilin protein family. Sema4A binds Tim-2, a member of the T cell, immunoglobulin and mucin (Tim) domain protein family expressed on activated T cells [27]. Sema 4D binds CD72, a member of the C-type lectin family, and uses it for its effects in lymphoid tissues [27]. Sema, semaphorin; PSI, plexin-semaphorin-integrin; IPT, immunoglobulin-like fold shared by plexins and transcription factors; GAP, GTPase-activating protein; MAM, Meprin, A5, Mu; PMR, polymorphic region; ITIM, immunoreceptor tyrosine-based inhibitory motif; IgV, immunoglobulin variable region.
The signal transduction cascades used by semaphorins are poorly understood. No canonical signal transduction pathways seem to mediate the effects of semaphorins, making the identification of semaphorin signaling intermediates difficult. Over the past few years, however, a number of proteins have been identified and linked with semaphorin signaling, including G proteins, kinases, regulators of cyclic nucleotide levels, oxidation-reduction enzymes, and regulators of the actin cytoskeleton (Table 2). These intermediates suggest that novel signaling cascades implement semaphorin function (reviewed in [21,41-44]), although a complete signaling pathway through which these proteins direct semaphorin function has not yet been characterized. Furthermore, semaphorin signaling intermediates have been identified using several different functional assays, complicating a precise determination of the roles of these proteins in the different semaphorin functions.
At the moment, the best characterized semaphorin signaling cascades are those used for axon guidance and cell migration. Semaphorin-mediated repulsive axon-guidance signaling depends on the large cytoplasmic domains of plexins, at least some of which have GTPase-activating protein (GAP) activity: these domains show sequence similarity to a group of Ras-family-specific GAPs, and mammalian PlexA1 and PlexB1 have GAP activity towards R-Ras [45,46]. The cytoplasmic domains of plexins also bind other small GTPases as well as binding regulators of GTPase activity, including guanine-nucleotide exchange factors (GEFs) and GAPs [44]. The functional implications of these interactions are best understood for mammalian Sema4D and mammalian PlexB1: activation of PlexB1 by Sema4D enhances the activity of RhoGEFs, activating the small GTPase RhoA, and leads to cytoskeletal rearrangement and repulsive axon guidance. There may be variation, however, in the signaling cascades activated by the different semaphorins. Repulsive axon guidance signaling by invertebrate Sema-1a or vertebrate Sema3A through class A plexins, for example, uses many proteins not currently characterized as important for repulsive axon guidance by Sema4D and PlexB1 [18,21,41,42].
Specific signaling proteins may also be required for the distinct functions of semaphorins. For example, Sema4D, together with PlexB1, limits cell migration or axon outgrowth by signaling through signaling proteins including the epidermal growth factor receptor ErbB2, Rho kinase, 12-15 lipoxygenase, and PlexC1; whereas Sema4D signaling through PlexB2 and the hepatocyte growth factor receptor Met, the receptor tyrosine kinase Ron, p190RhoGap, the tyrosine kinases Pyk2, Src, and Akt, and phosphatidylinositol 3-kinase enables cell migration or axon outgrowth (reviewed in [41,47]).
Importantly, recent work has also begun to identify mechanisms by which semaphorin signaling and its functional effects can be modulated. Neurotrophins, growth factors, chemokines, cell adhesion molecules, and integrins have all been shown to modulate semaphorin signaling, and some of these effects seem to occur through cyclic nucleotides, nitric oxide, and semaphorin receptor endocytosis [21,41,42]. Interestingly, semaphorins can also serve as cell-surface receptors for plexins and perhaps other proteins, and mediate some of their functional effects through 'reverse signaling' [48] (Table 2). In particular, transmembrane semaphorins can function as receptors essential for generating proper neuronal connectivity [49,50] and cardiac development [48], and these effects have been linked to the association of their cytoplasmic portions with signaling and anchoring proteins (Table 2).
Frontiers
Despite considerable progress in our characterization of members of the semaphorin family, much remains to be learned about their functions and molecular mechanisms of action. Several semaphorins have yet to be functionally characterized, and many have undergone only a cursory examination. A number of questions remain, including the purpose of having so many related semaphorins and the underlying logic to their complex expression patterns and physiological roles. The degree of interaction among semaphorins is also poorly understood. Do they regulate each other's signaling cascades? Do they physically associate? What special attributes and abilities do the secreted, transmembrane, and GPI-linked forms of semaphorins functionally provide?
Understanding the signaling cascades that underlie the different functional effects of semaphorins will provide insights into these important proteins. Are there differences in the signaling cascades activated by the different semaphorins? How much do their signaling cascades vary in order to mediate their different cellular effects? How do semaphorins exert their dramatic effects on the cytoskeleton?
A more detailed understanding of the role of semaphorins in the normal functioning adult is important. In the nervous system, the role of semaphorins in forming neural connections is well established, but the role of semaphorins in neural connectivity as it pertains to thought, emotion, memory, and behavior is unknown. The role of semaphorins in human disease and pathology is also poorly understood. Mutations in semaphorins are associated with patients with cancer [28], retinal degeneration [51], decreased bone mineral density [52], rheumatoid arthritis [53], and CHARGE syndrome (a disorder characterized by cranial nerve dysfunction, cardiac anomalies, and growth retardation) [54]. Further characterization of the semaphorins and a better understanding of their signaling mechanisms will undoubtedly uncover additional roles for semaphorins and semaphorin signaling in human disease.
Given the role of semaphorins in a wide range of tissues and functions including neurobiology, vasculobiology, cancer biology, and immunobiology, further characterizing the semaphorins and their signaling cascades will reveal fundamental mechanisms of how these systems work and strategies for preventing and treating pathologies associated with them.
Additional data files
The following additional data files are available: tables of the protein sequence identities between different semaphorins over the whole sequence (Additional data file 1) and the sema domain (Additional data file 2).
Supplementary Material
A table of the protein sequence identities between different semaphorins over the whole sequence
A table of the protein sequence identities between different semaphorins over the sema domain
Acknowledgments
Acknowledgements
We thank R. Giger, M. Henkemeyer, and A. Kolodkin for helpful comments on the manuscript, and Zhiyu Huang for helpful discussions. This work was supported by grants from the NIH/NIMH (MH069787), The Whitehall Foundation, and The March of Dimes Basil O'Connor Starter Scholar Research Award to J.R.T. J.R.T. is the Rita C. and William P. Clements, Jr Scholar in Medical Research.
References
- Kolodkin AL, Matthes D, O'Connor T, Patel NH, Admon A, Bentley D, Goodman CS. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-L. [DOI] [PubMed] [Google Scholar]
- Yamada A, Kubo K, Takeshita T, Harashima N, Kawano K, Mine T, Sagawa K, Sugamura K, Itoh K. Molecular cloning of a glycosylphosphatidylinositol-anchored molecule CDw108. J Immunol. 1999;162:4094–4100. [PubMed] [Google Scholar]
- Angelisova P, Drbal K, Cerny J, Hilgert I, Horejsi V. Characterization of the human leukocyte GPI-anchored glycoprotein CDw108 and its relation to other similar molecules. Immunobiology. 1999;200:234–245. doi: 10.1016/s0171-2985(99)80073-4. [DOI] [PubMed] [Google Scholar]
- Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992;148:318–323. [PubMed] [Google Scholar]
- Kolodkin AL, Matthes D, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-Z. [DOI] [PubMed] [Google Scholar]
- Semaphorin Nomenclature Committee Unified nomenclature for the semaphorins/collapsins. Cell. 1999;97:551–552. doi: 10.1016/S0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Love CA, Esnouf RM, Jones EY. The Sema domain. Curr Opin Struct Biol. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Koppel AM, Feiner L, Kobayashi H, Raper JA. A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron. 1997;19:531–537. doi: 10.1016/S0896-6273(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Eickholt BJ, Morrow R, Walsh FS, Doherty P. Structural features of collapsin required for biological activity and distribution of binding sites in the developing chick. Mol Cell Neurosci. 1997;9:358–371. doi: 10.1006/mcne.1997.0636. [DOI] [PubMed] [Google Scholar]
- Oster SF, Bodeker MO, He F, Sretavan DW. Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development. 2003;130:775–784. doi: 10.1242/dev.00299. [DOI] [PubMed] [Google Scholar]
- Behar O, Mizuno K, Badminton M, Woolf CJ. Semaphorin 3A growth cone collapse requires a sequence homologous to tarantula hanatoxin. Proc Natl Acad Sci USA. 1999;96:13501–13505. doi: 10.1073/pnas.96.23.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Araj HH, Ralls SA, Kolodkin AL. The transmembrane semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20:207–220. doi: 10.1016/S0896-6273(00)80450-X. [DOI] [PubMed] [Google Scholar]
- Kusy S, Funkelstein L, Bourgais D, Drabkin H, Rougon G, Roche J, Castellani V. Redundant functions but temporal and regional regulation of two alternatively spliced isoforms of semaphorin 3F in the nervous system. Mol Cell Neurosci. 2003;24:409–418. doi: 10.1016/S1044-7431(03)00197-0. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lutz B, Gertler F, Behl C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J Biol Chem. 2000;275:39647–39653. doi: 10.1074/jbc.M006316200. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Fiore R, Puschel AW. The function of semaphorins during nervous system development. Front Biosci. 2003;8:s484–s499. doi: 10.2741/1080. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Rice DS, Huang W, Jones HA, Hansen G, Ye GL, Xu N, Wilson EA, Troughton K, Vaddi K, Newton RC, et al. Severe retinal degeneration associated with disruption of semaphorin 4A. Invest Ophthalmol Vis Sci. 2004;45:2767–2777. doi: 10.1167/iovs.04-0020. [DOI] [PubMed] [Google Scholar]
- Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci. 2005;25:3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/S0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71:249–267. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegahara N, Kumanogoh A, Kikutani H. Semaphorins: a new class of immunoregulatory molecules. Philos Trans R Soc Lond B Biol Sci. 2005;360:1673–1680. doi: 10.1098/rstb.2005.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751–760. doi: 10.2741/1569. [DOI] [PubMed] [Google Scholar]
- Autiero M, De Smet F, Claes F, Carmeliet P. Role of neural guidance signals in blood vessel navigation. Cardiovasc Res. 2005;65:629–638. doi: 10.1016/j.cardiores.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174:51–59. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- Chabbert-de Ponnat I, Marie-Cardine A, Pasterkamp RJ, Schiavon V, Tamagnone L, Thomasset N, Bensussan A, Boumsell L. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int Immunol. 2005;17:439–447. doi: 10.1093/intimm/dxh224. [DOI] [PubMed] [Google Scholar]
- Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lohrum M, Adams RH, Puschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- Koppel AM, Raper JA. Collapsin-1 covalently dimerizes, and dimerization is necessary for collapsing activity. J Biol Chem. 1998;273:15708–15713. doi: 10.1074/jbc.273.25.15708. [DOI] [PubMed] [Google Scholar]
- Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J Immunol. 2001;166:4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- Eckhardt F, Behar O, Calautti E, Yonezawa K, Nishimoto I, Fishman MC. A novel transmembrane semaphorin can bind c-src. Mol Cell Neurosci. 1997;9:409–419. doi: 10.1006/mcne.1997.0644. [DOI] [PubMed] [Google Scholar]
- Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci. 2000;20:2638–2648. doi: 10.1523/JNEUROSCI.20-07-02638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363–1371. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Rougon G. Control of semaphorin signaling. Curr Opin Neurobiol. 2002;12:532–541. doi: 10.1016/S0959-4388(02)00357-4. [DOI] [PubMed] [Google Scholar]
- Ventura A, Pelicci PG. Semaphorins: green light for redox signaling? Sci STKE. 2002;2002:PE44. doi: 10.1126/stke.2002.155.pe44. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ. R-Ras fills another GAP in semaphorin signalling. Trends Cell Biol. 2005;15:61–64. doi: 10.1016/j.tcb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphy RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Abid A, Ismail M, Mehdi SQ, Khaliq S. Identification of novel mutations in SEMA4A gene associated with retinal degenerative diseases. J Med Genet. 2005 doi: 10.1136/jmg.2005.035055. doi:10.1136/jmg.2005.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JM, Oh B, Lee JY, Lee JK, Kimm K, Kim GS, Park BL, Cheong HS, Shin HD, Hong JM, et al. Association study of semaphorin 7a (sema7a) polymorphisms with bone mineral density and fracture risk in postmenopausal Korean women. J Hum Genet. 2006;51:112–117. doi: 10.1007/s10038-005-0331-z. [DOI] [PubMed] [Google Scholar]
- Miller LE, Weidler C, Falk W, Angele P, Schaumburger J, Scholmerich J, Straub RH. Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1156–1163. doi: 10.1002/art.20110. [DOI] [PubMed] [Google Scholar]
- Lalani SR, Safiullah AM, Molinari LM, Fernbach SD, Martin DM, Belmont JW. SEMA3E mutation in a patient with CHARGE syndrome. J Med Genet. 2004;41:e94. doi: 10.1136/jmg.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare N, Fascetti N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. Expression patterns of two new members of the semaphorin family in Drosophila suggest early functions during embryogenesis. Mech Dev. 2000;91:393–397. doi: 10.1016/S0925-4773(99)00297-X. [DOI] [PubMed] [Google Scholar]
- Giordano A, Coppari R, Castellucci M, Cinti S. Sema3a is produced by brown adipocytes and its secretion is reduced following cold acclimation. J Neurocytol. 2001;30:5–10. doi: 10.1023/A:1011916822633. [DOI] [PubMed] [Google Scholar]
- Giordano A, Cesari P, Capparuccia L, Castellucci M, Cinti S. Sema3A and neuropilin-1 expression and distribution in rat white adipose tissue. J Neurocytol. 2003;32:345–352. doi: 10.1023/B:NEUR.0000011328.61376.bb. [DOI] [PubMed] [Google Scholar]
- Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J Comp Neurol. 1995;361:321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- Rieger J, Wick W, Weller M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia. 2003;42:379–389. doi: 10.1002/glia.10210. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Wolfer DP, De Wit GMJ, Verhaagen J. Anatomy of rat semaphorin III/collapsin-1 mRNA expression and relationship to developing nerve tracts during neuroembryogenesis. J Comp Neurol. 1996;375:378–392. doi: 10.1002/(SICI)1096-9861(19961118)375:3<378::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Villegas G, Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev. 2002;119 Suppl 1:S149–S153. doi: 10.1016/S0925-4773(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Franssen EH, Ehlert EM, Taniguchi M, Verhaagen J. Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth. Mol Cell Neurosci. 2003;24:902–912. doi: 10.1016/S1044-7431(03)00243-4. [DOI] [PubMed] [Google Scholar]
- Marzioni D, Tamagnone L, Capparuccia L, Marchini C, Amici A, Todros T, Bischof P, Neidhart S, Grenningloh G, Castellucci M. Restricted innervation of uterus and placenta during pregnancy: evidence for a role of the repelling signal Semaphorin 3A. Dev Dyn. 2004;231:839–848. doi: 10.1002/dvdy.20178. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ, Ruitenberg M-J, Holtmaat AJGD, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- Loes S, Kettunen P, Kvinnsland IH, Taniguchi M, Fujisawa H, Luukko K. Expression of class 3 semaphorins and neuropilin receptors in the developing mouse tooth. Mech Dev. 2001;101:191–194. doi: 10.1016/S0925-4773(00)00545-1. [DOI] [PubMed] [Google Scholar]
- de Lange R, Dimoudis N, Weidle UH. Identification of genes associated with enhanced metastasis of a large cell lung carcinoma cell line. Anticancer Res. 2003;23:187–194. [PubMed] [Google Scholar]
- Puschel AW, Adams RH, Betz H. The sensory innervation of the mouse spinal cord may be patterned by differential expression of and differential responsiveness to semaphorins. Mol Cell Neurosci. 1996;7:419–431. doi: 10.1006/mcne.1996.0030. [DOI] [PubMed] [Google Scholar]
- Morris JS, Stein T, Pringle MA, Davies CR, Weber-Hall S, Ferrier RK, Bell AK, Heath VJ, Gusterson BA. Involvement of axonal guidance proteins and their signaling partners in the developing mouse mammary gland. J Cell Physiol. 2006;206:16–24. doi: 10.1002/jcp.20427. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Fried K. Neurites from trigeminal ganglion explants grown in vitro are repelled or attracted by tooth-related tissues depending on developmental stage. Neuroscience. 2004;125:149–161. doi: 10.1016/j.neuroscience.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Cohen RI, Rottkamp DM, Maric D, Barker JL, Hudson LD. A role for semaphorins and neuropilins in oligodendrocyte guidance. J Neurochem. 2003;85:1262–1278. doi: 10.1046/j.1471-4159.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- Kagoshima M, Ito T. Diverse gene expression and function of semaphorins in developing lung: positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes Cells. 2001;6:559–571. doi: 10.1046/j.1365-2443.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper J. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14:1131–1140. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Severance SM, Yee CS, Gemza DL, Raper JA, Kuwada JY. Analysis of a zebrafish semaphorin reveals potential functions in vivo. Dev Dyn. 1999;214:13–25. doi: 10.1002/(SICI)1097-0177(199901)214:1<13::AID-DVDY2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lallier TE. Semaphorin profiling of periodontal fibroblasts and osteoblasts. J Dent Res. 2004;83:677–682. doi: 10.1177/154405910408300904. [DOI] [PubMed] [Google Scholar]
- Jin Z, Chau MD, Bao ZZ. Sema3D, Sema3F, and Sema5A are expressed in overlapping and distinct patterns in chick embryonic heart. Dev Dyn. 2006;235:163–169. doi: 10.1002/dvdy.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CR, Klingelhofer J, Tarabykina S, Hulgaard EF, Kramerov D, Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58:1238–1244. [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Takeda N, Inoue T, Kubo T, Inagaki S. Expression of mouse semaphorin H mRNA in the inner ear of mouse fetuses. Neurosci Lett. 1999;261:127–129. doi: 10.1016/S0304-3940(98)00988-4. [DOI] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Sakai T, Fujioka S, Mori T, Ohoka Y, Takeda N, Kubo T, Inagaki S. Developmental localization of semaphorin H messenger RNA acting as a collapsing factor on sensory axons in the mouse brain. Neuroscience. 1999;93:401–408. doi: 10.1016/S0306-4522(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Sekido Y, Bader S, Latif F, Chen J-Y, Duh F-M, Wei M-H, Albanesi JP, Lee C-C, Lerman MI, Minna JD. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci USA. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Urquhart ER, Gillespie SKH, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: Insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–1092. doi: 10.1016/S0896-6273(00)80625-X. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Masuda T, Fukaya M, Kataoka H, Mishina M, Yaginuma H, Watanabe M, Shimizu T. Identification and characterization of a novel member of murine semaphorin family. Genes Cells. 2005;10:785–792. doi: 10.1111/j.1365-2443.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, Kikutani H. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Gautier G, de Saint-Vis B, Senechal B, Pin JJ, Bates EE, Caux C, Geissmann F, Garrone P. The class 6 semaphorin SEMA6A is induced by interferon-gamma and defines an activation status of langerhans cells observed in pathological situations. Am J Pathol. 2006;168:453–465. doi: 10.2353/ajpath.2006.050288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt C, Muller M, Badde A, Garner CC, Gundelfinger ED, Puschel AW. Semaphorin 4B interacts with the post-synaptic density protein PSD-95/SAP90 and is recruited to synapses through a C-terminal PDZ-binding motif. FEBS Lett. 2005;579:3821–3828. doi: 10.1016/j.febslet.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Furuyama T, Iwahashi Y. Identification of a member of mouse semaphorin family. FEBS Letters. 1995;370:269–272. doi: 10.1016/0014-5793(95)00850-9. [DOI] [PubMed] [Google Scholar]
- Ko JA, Gondo T, Inagaki S, Inui M. Requirement of the transmembrane semaphorin Sema4C for myogenic differentiation. FEBS Lett. 2005;579:2236–2242. doi: 10.1016/j.febslet.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Ohoka Y, Sugimoto H, Fujioka S, Amazaki M, Kurinami H, Miyazaki N, Tohyama M, Furuyama T. Sema4c, a transmembrane semaphorin, interacts with a post-synaptic density protein, PSD-95. J Biol Chem. 2001;276:9174–9181. doi: 10.1074/jbc.M009051200. [DOI] [PubMed] [Google Scholar]
- Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA. 1996;93:11780–11785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Inagaki S, Kosugi A, Noda S, Saitoh S, Ogata M, Iwahashi Y, Miyazaki N, Hamaoka T, Tohyama M. Identification of a novel transmembrane semaphorin expressed on lymphocytes. J Biol Chem. 1996;271:33376–33381. doi: 10.1074/jbc.271.52.33376. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Severance SM, Yee CS, Gemza DL, Kuwada JY. Molecular cloning and expression of two novel zebrafish semaphorins. Mech Dev. 1998;76:165–168. doi: 10.1016/S0925-4773(98)00124-5. [DOI] [PubMed] [Google Scholar]
- Encinas JA, Kikuchi K, Chedotal A, de Castro F, Goodman CS, Kimura T. Cloning, expression, and genetic mapping of Sema W, a member of the semaphorin family. Proc Natl Acad Sci USA. 1999;96:2491–2496. doi: 10.1073/pnas.96.5.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze W, Eulenburg V, Lessmann V, Herrmann L, Dittmar T, Gundelfinger ED, Heumann R, Erdmann KS. Semaphorin4F interacts with the synapse-associated protein SAP90/PSD-95. J Neurochem. 2001;78:482–489. doi: 10.1046/j.1471-4159.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Li H, Wu DK, Sullivan SL. Characterization and expression of sema4g, a novel member of the semaphorin gene family. Mech Dev. 1999;87:169–173. doi: 10.1016/S0925-4773(99)00125-2. [DOI] [PubMed] [Google Scholar]
- Woodhouse EC, Fisher A, Bandle RW, Bryant-Greenwood B, Charboneau L, Petricoin EF, 3rd, Liotta LA. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proc Natl Acad Sci USA. 2003;100:11463–11468. doi: 10.1073/pnas.2031202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Betz H, Puschel AW. A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev. 1996;57:33–45. doi: 10.1016/0925-4773(96)00525-4. [DOI] [PubMed] [Google Scholar]
- Bahri SM, Chia W, Yang X. Characterization and mutant analysis of the Drosophila sema 5c gene. Dev Dyn. 2001;221:322–330. doi: 10.1002/dvdy.1142. [DOI] [PubMed] [Google Scholar]
- Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, Macdougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, et al. Recombinant semaphorin 6a-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther. 2005;4:659–668. doi: 10.1158/1535-7163.MCT-04-0290. [DOI] [PubMed] [Google Scholar]
- Zhou L, White FA, Lentz SI, Wright DE, Fisher DA, Snider WD. Cloning and expression of a novel murine semaphorin with structural similarity to insect semaphorin I. Mol Cell Neurosci. 1997;9:26–41. doi: 10.1006/mcne.1997.0607. [DOI] [PubMed] [Google Scholar]
- Collet P, Domenjoud L, Devignes MD, Murad H, Schohn H, Dauca M. The human semaphorin 6B gene is down regulated by PPARs. Genomics. 2004;83:1141–1150. doi: 10.1016/j.ygeno.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kury P, Abankwa D, Kruse F, Greiner-Petter R, Muller HW. Gene expression profiling reveals multiple novel intrinsic and extrinsic factors associated with axonal regeneration failure. Eur J Neurosci. 2004;19:32–42. doi: 10.1111/j.1460-9568.2004.03112.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Chedotal A, Hanafusa H, Ujimasa Y, de Castro F, Goodman CS, Kimura T. Cloning and characterization of a novel class VI semaphorin, semaphorin Y. Mol Cell Neurosci. 1999;13:9–23. doi: 10.1006/mcne.1998.0732. [DOI] [PubMed] [Google Scholar]
- Qu X, Wei H, Zhai Y, Que H, Chen Q, Tang F, Wu Y, Xing G, Zhu Y, Liu S, et al. Identification, characterization, and functional study of the two novel human members of the semaphorin gene family. J Biol Chem. 2002;277:35574–35585. doi: 10.1074/jbc.M206451200. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Shimizu T. Characterization of a novel member of murine semaphorin family. Biochem Biophys Res Commun. 2004;314:242–248. doi: 10.1016/j.bbrc.2003.12.083. [DOI] [PubMed] [Google Scholar]
- Xu X, Ng S, Wu Z-L, Nguyen D, Homburger S, Seidel-Dugan C, Ebens A, Luo Y. Human Semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J Biol Chem. 1998;273:22428–22434. doi: 10.1074/jbc.273.35.22428. [DOI] [PubMed] [Google Scholar]
- Delorme G, Saltel F, Bonnelye E, Jurdic P, Machuca-Gayet I. Expression and function of semaphorin 7A in bone cells. Biol Cell. 2005;97:589–597. doi: 10.1042/BC20040103. [DOI] [PubMed] [Google Scholar]
- Bobolis KA, Moulds JJ, Telen MJ. Isolation of the JMH antigen on a novel phosphatidylinositol-linked human membrane protein. Blood. 1992;79:1574–1581. [PubMed] [Google Scholar]
- Ginzburg VE, Roy PJ, Culotti JG. Semaphorin 1a and semaphorin 1b are required for correct epidermal cell positioning and adhesion during morphogenesis in C. elegans. Development. 2002;129:2065–2078. doi: 10.1242/dev.129.9.2065. [DOI] [PubMed] [Google Scholar]
- Roy PJ, Zheng H, Warren CE, Culotti JG. mab-20 encodes Semaphorin-2a and is required to prevent ectopic cell contacts during epidermal morphogenesis in Caenorhabditis elegans. Development. 2000;127:755–767. doi: 10.1242/dev.127.4.755. [DOI] [PubMed] [Google Scholar]
- Matthes DJ, Sink H, Kolodkin AL, Goodman CS. Semaphorin II can function as a selective inhibitor of specific synaptic arborizations in Drosophila. Cell. 1995;81:631–639. doi: 10.1016/0092-8674(95)90084-5. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones, and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- Gagliardini V, Fankhauser C. Semaphorin III can induce death in sensory neurons. Mol Cell Neurosci. 1999;14:301–316. doi: 10.1006/mcne.1999.0787. [DOI] [PubMed] [Google Scholar]
- Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- Miao H-Q, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Osborne NJ, Begbie J, Chilton JK, Schmidt H, Eickholt BJ. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev Dyn. 2005;232:939–949. doi: 10.1002/dvdy.20258. [DOI] [PubMed] [Google Scholar]
- Catalano A, Caprari P, Rodilossi S, Betta P, Castellucci M, Casazza A, Tamagnone L, Procopio A. Cross-talk between vascular endothelial growth factor and semaphorin-3A pathway in the regulation of normal and malignant mesothelial cell proliferation. FASEB J. 2004;18:358–360. doi: 10.1096/fj.03-0513fje. [DOI] [PubMed] [Google Scholar]
- Ito T, Kagoshima M, Sasaki Y, Li C, Udaka N, Kitsukawa T, Fujisawa H, Taniguchi M, Yagi T, Kitamura H, Goshima Y. Repulsive axon guidance molecule Sema3A inhibits branching morphogenesis of fetal mouse lung. Mech Dev. 2000;97:35–45. doi: 10.1016/S0925-4773(00)00401-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/S0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, Klagsbrun M, Mamluk R, Newgreen DF. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/S0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Tomizawa Y, Sekido Y, Kondo M, Gao B, Yokota J, Roche J, Drabkin H, Lerman MI, Gazdar AF, Minna JD. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci USA. 2001;98:13954–13959. doi: 10.1073/pnas.231490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nakamura F, Jin Z, Kalb R, Strittmatter S. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48:63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- Moreno-Flores MT, Martin-Aparicio E, Martin-Bermejo MJ, Agudo M, McMahon S, Avila J, Diaz-Nido J, Wandosell F. Semaphorin 3C preserves survival and induces neuritogenesis of cerebellar granule neurons in culture. J Neurochem. 2003;87:879–890. doi: 10.1046/j.1471-4159.2003.02051.x. [DOI] [PubMed] [Google Scholar]
- Sakai T, Furuyama T, Ohoka Y, Miyazaki N, Fujioka S, Sugimoto H, Amasaki M, Hattori S, Matsuya T, Inagaki S. Mouse semaphorin H induces PC12 cell neurite outgrowth activating Ras-mitogen-activated protein kinase signaling pathway via Ca(2+) influx. J Biol Chem. 1999;274:29666–29671. doi: 10.1074/jbc.274.42.29666. [DOI] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008–1015. doi: 10.1158/0008-5472.CAN-03-3090. [DOI] [PubMed] [Google Scholar]
- Nasarre P, Constantin B, Rouhaud L, Harnois T, Raymond G, Drabkin HA, Bourmeyster N, Roche J. Semaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreading. Neoplasia. 2003;5:83–92. doi: 10.1016/s1476-5586(03)80020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, de Castro F, Le Bras B, Heydon K, Queraud-LeSaux F, Bloch-Gallego E, Chedotal A, Zalc B, Thomas JL. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22:5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Moens CB. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev Biol. 2005;280:373–385. doi: 10.1016/j.ydbio.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Pozas E, Pascual M, Nguyen Ba-Charvet KT, Guijarro P, Sotelo C, Chedotal A, Del Rio JA, Soriano E. Age-dependent effects of secreted Semaphorins 3A, 3F, and 3E on developing hip-pocampal axons: in vitro effects and phenotype of Semaphorin 3A (-/-) mice. Mol Cell Neurosci. 2001;18:26–43. doi: 10.1006/mcne.2001.0999. [DOI] [PubMed] [Google Scholar]
- Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–6680. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, non-metastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI200421378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang R, Davalos AR, Hensel CH, Zhou XJ, Tse C, Naylor SL. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res. 2002;62:2637–2643. [PubMed] [Google Scholar]
- Yukawa K, Tanaka T, Bai T, Ueyama T, Owada-Makabe K, Tsubota Y, Maeda M, Suzuki K, Kikutani H, Kumanogoh A. Semaphorin 4A induces growth cone collapse of hippocampal neurons in a Rho/Rho-kinase-dependent manner. Int J Mol Med. 2005;16:115–118. [PubMed] [Google Scholar]
- Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold C, Elhabazi A, Bismuth G, Bensussan A, Boumsell L. CD100 is associated with CD45 at the surface of human T lymphocytes. Role in T cell homotypic adhesion. J Immunol. 1996;157:5262–5268. [PubMed] [Google Scholar]
- Giraudon P, Vincent P, Vuaillat C, Verlaeten O, Cartier L, Marie-Cardine A, Mutin M, Bensussan A, Belin MF, Boumsell L. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J Immunol. 2004;172:1246–1255. doi: 10.4049/jimmunol.172.2.1246. [DOI] [PubMed] [Google Scholar]
- Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/S1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Masuda K, Toguchi M, Ohoka Y, Sakai T, Furuyama T, Inagaki S. Neurotrophic effect of Semaphorin 4D in PC12 cells. Biochem Biophys Res Commun. 2003;301:304–310. doi: 10.1016/S0006-291X(02)03023-1. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/S0896-6273(02)00750-X. [DOI] [PubMed] [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- Xiao T, Shoji W, Zhou W, Su F, Kuwada JY. Transmembrane sema4E guides branchiomotor axons to their targets in zebrafish. J Neurosci. 2003;23:4190–4198. doi: 10.1523/JNEUROSCI.23-10-04190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R, Rahim B, Christoffels VM, Moorman AF, Puschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol. 2005;25:2310–2319. doi: 10.1128/MCB.25.6.2310-2319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25:3628–3637. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K, Fox J, Deen K, Pettman G, et al. Sema7A is a potent monocyte stimulator. Scand J Immunol. 2002;56:270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Gardner JD, Tscharke DC, Reading PC, Smith GL. Vaccinia virus semaphorin A39R is a 50-55 kDa secreted glycoprotein that affects the outcome of infection in a murine intradermal model. J Gen Virol. 2001;82:2083–2093. doi: 10.1099/0022-1317-82-9-2083. [DOI] [PubMed] [Google Scholar]
- Comeau MR, Johnson R, DuBose RF, Peterson M, Gearing P, VandenBos T, Park L, Farrah T, Buller RM, Cohen JI, et al. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity. 1998;8:473–482. doi: 10.1016/S1074-7613(00)80552-X. [DOI] [PubMed] [Google Scholar]
- Walzer T, Galibert L, De Smedt T. Poxvirus semaphorin A39R inhibits phagocytosis by dendritic cells and neutrophils. Eur J Immunol. 2005;35:391–398. doi: 10.1002/eji.200425669. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/S0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Fujii T, Nakao F, Shibata Y, Shioi G, Kodama E, Fujisawa H, Takagi S. Caenorhabditis elegans PlexinA, PLX-1, interacts with transmembrane semaphorins and regulates epidermal morphogenesis. Development. 2002;129:2053–2063. doi: 10.1242/dev.129.9.2053. [DOI] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/S0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/S0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/S0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–545. doi: 10.1016/S0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming G-L, Song H-J, Chedotal A, Winberg ML, Goodman CS, Poo M-M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/S0092-8674(00)80063-X. [DOI] [PubMed] [Google Scholar]
- Masuda K, Furuyama T, Takahara M, Fujioka S, Kurinami H, Inagaki S. Sema4D stimulates axonal outgrowth of embryonic DRG sensory neurones. Genes Cells. 2004;9:821–829. doi: 10.1111/j.1365-2443.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/S1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Tamagnone L, Bai J, Comoglio PM, Montell D, Goodman CS. The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron. 2001;32:53–62. doi: 10.1016/S0896-6273(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Ayoob JC, Yu HH, Terman JR, Kolodkin AL. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci. 2004;24:6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/S0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Terman JR, Kolodkin AL. Nervy links protein kinase A to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- Dalpe G, Brown L, Culotti JG. Vulva morphogenesis involves attraction of plexin 1-expressing primordial vulva cells to semaphorin 1a sequentially expressed at the vulva midline. Development. 2005;132:1387–1400. doi: 10.1242/dev.01694. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron. 2001;32:249–263. doi: 10.1016/S0896-6273(01)00478-0. [DOI] [PubMed] [Google Scholar]
- Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45:513–523. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Sainturet N, Meyronet D, Perraut M, Miehe M, Roussel G, Aunis D, Belin MF, Thomasset N. Differential MAP kinases activation during semaphorin3A-induced repulsion or apoptosis of neural progenitor cells. Mol Cell Neurosci. 2004;25:722–731. doi: 10.1016/j.mcn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals crosstalk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/S0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Brown M, Jacobs T, Eickholt B, Ferrari G, Teo M, Monfries C, Qi RZ, Leung T, Lim L, Hall C. Alpha2-chimaerin, cyclin-dependent kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J Neurosci. 2004;24:8994–9004. doi: 10.1523/JNEUROSCI.3184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of Rac1. J Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–920. doi: 10.1016/S0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Werner M, Heppenstall PA, Henning M, More MI, Kuhbandner S, Lewin GR, Hofmann F, Feil R, Rathjen FG. cGMP-mediated signaling via cGKIalpha is required for the guidance and connectivity of sensory axons. J Cell Biol. 2002;159:489–498. doi: 10.1083/jcb.200207058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Mitsui N, Inatome R, Takahashi S, Goshima Y, Yamamura H, Yanagi S. Involvement of Fes/Fps tyrosine kinase in semaphorin3A signaling. EMBO J. 2002;21:3274–3285. doi: 10.1093/emboj/cdf328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol. 2002;157:211–217. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikule K, Gatlin JC, de la Houssaye BA, Pfenninger KH. Growth cone collapse induced by semaphorin 3A requires 12/15-lipoxygenase. J Neurosci. 2002;22:4932–4941. doi: 10.1523/JNEUROSCI.22-12-04932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci. 2006;119:951–957. doi: 10.1242/jcs.02801. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T cell signal transduction and function. Blood. 2005 doi: 10.1182/blood-2005-06-2445. doi:10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanata SM, Hovatta I, Rohm B, Puschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Nasarre P, Kusy S, Constantin B, Castellani V, Drabkin HA, Bagnard D, Roche J. Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin-mediated cell adhesion. Neoplasia. 2005;7:180–189. doi: 10.1593/neo.04481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Singh KK, Tessier-Lavigne M, Miller FD, Kaplan DR. Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase signaling: a mechanism for growth cone collapse. J Neurosci. 2003;23:7602–7609. doi: 10.1523/JNEUROSCI.23-20-07602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 2004;18:592–594. doi: 10.1096/fj.03-0957fje. [DOI] [PubMed] [Google Scholar]
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- Aurandt J, Li W, Guan KL. Semaphorin 4D activates the MAPK pathway downstream of plexin-B1. Biochem J. 2006;394:459–464. doi: 10.1042/BJ20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. doi: 10.1016/S0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Harada A, Negishi M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J Biol Chem. 2003;278:25671–25677. doi: 10.1074/jbc.M303047200. [DOI] [PubMed] [Google Scholar]
- Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Kalb RG, Strittmatter SM. A PDZ protein regulates the distribution of the transmembrane semaphorin, M-SemF. J Biol Chem. 1999;274:14137–14146. doi: 10.1074/jbc.274.20.14137. [DOI] [PubMed] [Google Scholar]
- Ohoka Y, Hirotani M, Sugimoto H, Fujioka S, Furuyama T, Inagaki S. Semaphorin 4C , a transmembrane semaphorin, [corrected] associates with a neurite-outgrowth-related protein, SFAP75. Biochem Biophys Res Commun. 2001;280:237–243. doi: 10.1006/bbrc.2000.4080. [DOI] [PubMed] [Google Scholar]
- Elhabazi A, Lang V, Herold F, Freeman GJ, Bensussan A, Boumsell L, Bismuth G. The human semaphorin-like leukocyte cell surface molecule CD100 associates with a serine kinase activity. J Biol Chem . 1997;272:23515–23520. doi: 10.1074/jbc.272.38.23515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A table of the protein sequence identities between different semaphorins over the whole sequence
A table of the protein sequence identities between different semaphorins over the sema domain


