Abstract
γ-Linolenic acid (GLA), a nutritionally important fatty acid in mammals, is synthesized by a Δ6 desaturase. Here, we report identification of PiD6, a new cDNA from the oleaginous fungus, Pythium irregulare, encoding a 459-amino acid protein that shares sequence similarity to carboxyl-directed desaturases from various species. Expression of PiD6 in yeast (Saccharomyces cerevisiae) revealed that it converts exogenously supplied linoleic acid into GLA, indicating that it encodes a Δ6 fatty acid desaturase. Expression of the desaturase in Brassica juncea under the control of the Brassica napus napin promoter resulted in production of three Δ6 unsaturated fatty acids (18:2–6, 9; 18:3–6, 9, 12; and 18:4–6, 9, 12, 15) in seeds. Among them, GLA (18:3–6, 9, 12) is the most abundant and accounts for up to 40% of the total seed fatty acids. Lipid class and positional analysis indicated that GLA is almost exclusively incorporated into triacylglycerol (98.5%) with only trace amounts found in the other lipids. Within triacylglycerols, GLA is more abundant at the sn-2 position.
In mammals and some plants and fungi, a Δ6 fatty acid desaturase is responsible for conversion of linoleic acid (LA; 18:2–9, 12) to γ-linolenic acid (GLA; 18:3–6, 9, 12) and of α-linolenic acid (ALA, 18:3–9, 12, 15) to stearidonic acid (SDA, 18:4–6, 9, 12, 15). GLA is a nutritionally important fatty acid in mammals. It is involved in membrane structure in various cells and in the biosynthesis of very long-chain polyunsaturated fatty acids, both in humans and animals (Gunstone, 1992; Huang and Milles, 1996). Dietary supplementation with GLA has been shown to reduce the risks of, or have positive effect on, those disorders that are associated with a low level of this fatty acid (Horrobin, 1990, 1992; Huang and Milles, 1996). As a result, there is considerable interest in the large-scale production of GLA in oilseed crops (Huang and Ziboh, 2001).
Fatty acid desaturation involves the regiospecific introduction of carbon-carbon double bonds into aliphatic acyl chains. According to localization and cofactor requirements, desaturases can be classified into two major groups. The soluble group, such as the plant plastid Δ9 desaturases, uses acyl carrier protein thioesters as substrates, and NADPH:ferredoxin oxidoreductase and ferredoxin as electron donors. The membrane-bound desaturases, such as the plant microsomal Δ12 desaturase, use fatty acids esterified to complex lipid as the substrate, and NADH:cytochrome (cyt) b5 oxidoreductase and cyt b5 as electron donors (Heinz, 1993; Shanklin and Cahoon, 1998).
Compared with the soluble desaturases, microsomal desaturases are more varied and evolutionarily diversified, and can be further divided into subgroups. The carboxyl-directed desaturases, also known as the front-end desaturases (Napier et al., 1997), “measure” distance from the carboxy terminus of a fatty acid and introduce a double bond between the existing double bond and the carboxy terminus of the fatty acyl chain (Girke et al., 1998). Examples of this type of desaturase are Δ4 (Qiu et al., 2001a), Δ5 (Knutzon et al., 1998; Michaelson et al., 1998a), and Δ6 (Sayanova et al., 1997; Napier et al., 1998; Cho et al., 1999) desaturases. The “methyl-directed desaturases,” on the other hand, measure distance from the methyl terminus of a fatty acid and introduce a double bond between the existing double bond and the methyl terminus of the fatty acyl chain. The examples of this type of desaturases are ω-3 desaturases from oilseed and Caenorhabditis elegans (Meesapyodsuk et al., 2000). Each type of desaturase possesses characteristic consensus protein sequence motifs. For instance, the methyl-directed desaturases contain three conserved His-rich motifs, whereas the carboxyl-directed desaturases usually contain, besides the three His-rich motifs, an extra heme-binding motif in an N-terminal cyt b5-like extension. Deletion of this extra domain or site mutagenesis of key amino acids in the motif resulted in disruption of the enzymatic function (Sayanova et al., 1999b; Qiu et al., 2002).
Polyunsaturated fatty acids are long-chain fatty acids containing two or more double bonds in their acyl chains. Biosynthesis of polyunsaturated fatty acids involves both methyl-directed and carboxyl-directed desaturases. The primary product of fatty acid biosynthesis in oilseed crops is the 18-carbon monounsaturate, oleic acid (18:1–9). Sequential desaturation of oleic acid and its elongated products at both ends by methyl- and carboxyl-directed desaturases results in various polyunsaturated fatty acids.
Pythium irregulare is an oleaginous fungus that is unusual in that it contains a large amount of arachidonic acid and eicosapentaenoic acid. Based on the information that microsomal carboxyl-directed desaturases have similar primary structure, we undertook a PCR approach with two degenerate primers targeting the heme-binding and the third His-rich motifs to clone genes that are involved in biosynthesis of the fatty acids in P. irregulare. One unique cDNA, PiD6, was identified (GenBank accession no. AF419296); it encodes a 459-amino acid protein that shares sequence similarity to carboxyl-directed desaturases from various species (Sayanova et al., 1997; Girke et al., 1998; Napier et al., 1998; Aki et al., 1999; Cho et al., 1999; Huang et al., 1999). Expression of PiD6 in yeast (Saccharomyces cerevisiae) revealed that it converts exogenously supplied LA into GLA, indicating that it encodes a Δ6 fatty acid desaturase. Expression of the desaturase in Brassica juncea under the control of the oilseed napin promoter resulted in production of three Δ6 unsaturated fatty acids (18:2–6, 9; 18:3–6, 9, 12; and 18:4–6, 9, 12, 15) in seeds. Among them, GLA is the most abundant and accounts for up to 40% of the total seed fatty acids of transgenic plants; SDA (18:4–6, 9, 12, 15) follows in a range of 3% to 10%. GLA is almost exclusively incorporated into triacylglycerols (TAGs) with a preference for the sn-2 position.
RESULTS
Identification of PiD6 from P. irregulare
To identify genes encoding Δ6 desaturases involved in the biosynthesis of polyunsaturated fatty acids in P. irregulare, a PCR-based cloning strategy was adopted. Two degenerate primers are designed to target sequences corresponding to the heme-binding motif of the cyt b5-like domain and the third His-rich motif in the microsomal desaturases. Using this strategy we identified a cDNA fragment from P. irregulare that encoded a partial amino acid sequence containing a cyt b5-like domain in the N terminus and having sequence similarity to carboxyl-directed desaturases from other various species.
To isolate the full-length cDNA clone, the insert was used as a probe to screen a cDNA library of P. irregulare, which resulted in the identification of several cDNA clones. Sequencing identified a full-length cDNA, PiD6. The open reading frame (ORF) of PiD6 is 1,380 bp and codes for 459 amino acids.
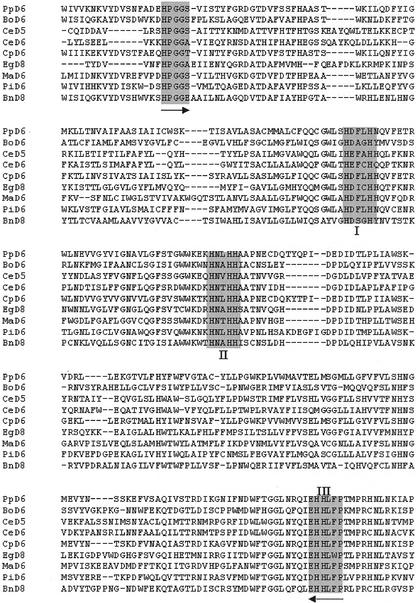
Sequence analysis indicates that the PiD6 protein shares high sequence similarity to the front-end desaturases and related enzymes from various sources, such as a Δ-8 sphingolipid desaturase from oilseed (Sperling et al., 1998), a bifunctional Δ6 acetylenase/desaturase from Ceratodon purpureus (Sperling et al., 2000), a Δ8 desaturase from Euglena (Euglena gracilis; Wallis and Browse, 1999), a Δ5 desaturase from C. elegans (Michaelson et al., 1998b), and Δ6 desaturases from C. elegans (Napier et al., 1998), borage (Borago officinalis; Sayanova et al., 1997), Mortierella alpina (Michaelson et al., 1998a), and Physcomitrella patens (Girke et al., 1998). Alignment of those sequences indicated that the identity occurs mainly in heme-binding motif of the cyt b5-like region and the three conserved His box areas (Fig. 1). It was noted that in the third His box of PiD6, Glu is replaced by Asp. Phylogenetic analysis of these sequences indicated that PiD6 clusters with the main group of the Δ6 desaturases from fungus and moss, which distinguish themselves from the Δ6 desaturases from animal and higher plants and from the desaturase-related enzymes (data not shown). These results suggest that PiD6 may be a Δ6 desaturase involved in the biosynthesis of GLA and SDA in P. irregulare.
Figure 1.
Alignment of PiD6 (ranging from 15 to 426 amino acids) and its orthologs by the Clustal method (Higgins and Sharp, 1989). The conservative motifs such as the cyt b5 heme binding and the three His boxes are highlighted. The two arrows indicate the binding locations of the two degenerate primers. BnD8, A Δ-8 sphingolipid desaturase from oilseed (Sperling et al., 1998); CpD6, a bifunctional Δ6 acetylenase/desaturase from C. purpureus (Sperling et al., 2000); EgD8, a Δ8 desaturase from Euglena (Wallis and Browse, 1999); CeD5, a Δ5 desaturase from C. elegans (Michaelson et al., 1998b); CeD6, a Δ6 desaturase from C. elegans (Napier et al., 1998); BoD6, a Δ6 desaturase from borage (Sayanova et al., 1997); MoD6, a Δ6 desaturase from M. alpina (Michaelson et al., 1998a); PpD6, a Δ6 desaturase from P. patens (Girke et al., 1998); PiD6, this report.
Expression of PiD6 in Yeast
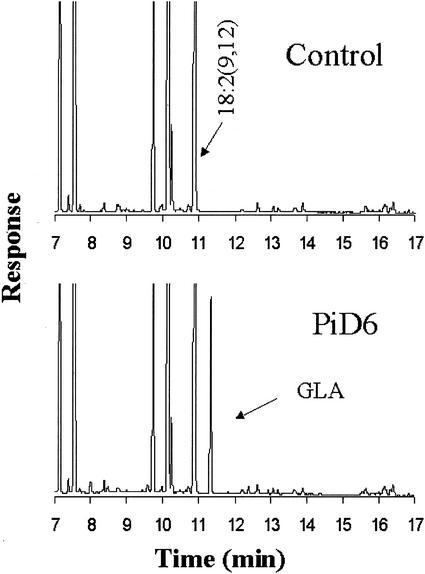
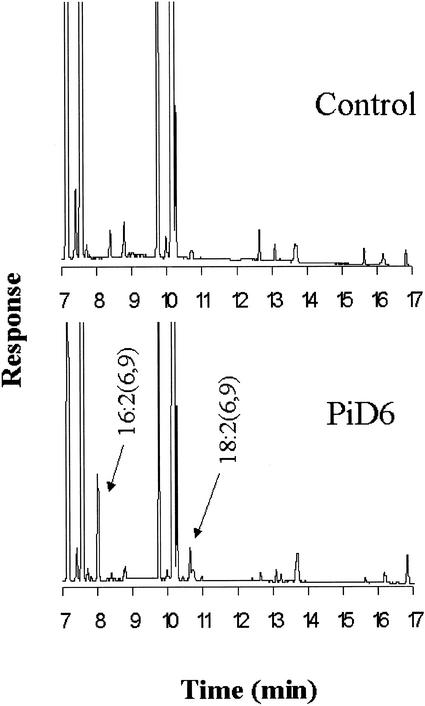
The yeast host strain Invsc1 was transformed with a plasmid containing the full-length ORF of PiD6 under the control of the Gal-inducible promoter GAL1. When the yeast transformant was induced by Gal in a medium containing LA, a prominent extra peak accounting for about 6% of the total fatty acids was observed in the chromatogram of the fatty acid methyl esters (FAMEs) accumulating in the transformants compared with the control (Fig. 2). A comparison of the chromatogram with that of FAME standards revealed that the peak has a retention time identical to the GLA standard. To confirm the regiochemistry of the products, the diethylamine derivatives of the fatty acid from the expressing strain were analyzed by gas chromatography (GC)-mass spectroscopy (MS). The spectrum was identical to the reference standard (data not shown). These results demonstrate that PiD6 is a Δ6 desaturase that converts LA to GLA in yeast. When the yeast transformant was induced by Gal in a medium without LA, two small peaks (approximately 1% of total) were detected in the transgenic FAME fraction that were determined to be 16:2(6, 9) and 18:2(6, 9), respectively (Fig. 3).
Figure 2.
GC analysis of FAMEs from yeast (strain Invsc1) expressing PiD6 cultured with exogenous LA.
Figure 3.
GC analysis of FAMEs from yeast (strain Invsc1) expressing PiD6 cultured without exogenous fatty acids.
Expression of PiD6 in B. juncea
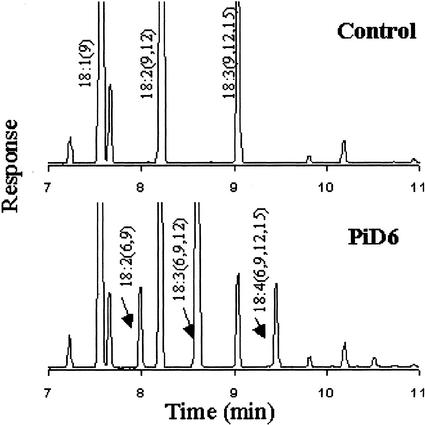
To produce Δ6-desaturated fatty acids in seeds of B. juncea, we transformed the B. juncea with the construct that contains PiD6 cDNA under the control of a heterologous seed-specific promoter (oilseed napin promoter). Eleven independent transgenic plants were obtained. Transformants were verified by PCR using two internal primers targeting the Δ6 desaturase. Fatty acid analysis of the transgenic seeds indicated that there were three new fatty acids in the gas chromatogram of most transgenics compared with the wild-type control (Fig. 4). The three fatty acids were identified as 18:2(6, 9); 18:3(6, 9, 12); and 18:4(6, 9, 12, 15). This result indicated that B. juncea could functionally express PiD6 from P. irregulare, introducing a double bond at position 6 of endogenous substrates 18:1(9); 18:2(9, 12); and 18:3(9, 12, 15), resulting in production of the corresponding Δ6 fatty acids in the transgenic seeds.
Figure 4.
GC analysis of seed FAMEs from B. juncea expressing PiD6. Three new peaks indicate three Δ6-desaturated fatty acids in transgenic seeds.
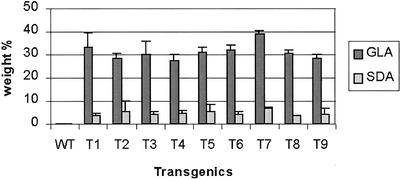
Among the three new fatty acids produced in transgenic seeds, GLA is the most abundant one, the level of which is in the range of 25% to 40% of the total fatty acids in transgenic seeds. The next most abundant component is SDA, which accounts for 2% to 10% of the total fatty acids in several transgenic lines (Fig. 5).
Figure 5.
Weight percentage of GLA and SDA accumulating in transgenic seeds of transgenic lines of Brassica juncea.
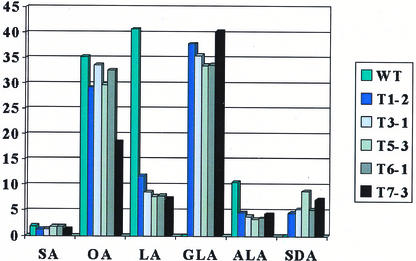
The fatty acid compositions of transgenic seeds are shown in Figure 6. It is clear that the high-level production of Δ6-desaturated fatty acids occurs at the cost of two major fatty acids, LA and linolenic acid. Proportions of oleic and stearic acids in transgenics are slightly but not significantly reduced compared with those in the wild-type control. The content of LA in the transgenics was dramatically reduced. In the untransformed wild type, LA accounts for more than 40% of the total fatty acids in seeds. In transgenics, the level was reduced to less than 10%.
Figure 6.
Fatty acid compositions of the seed lipids from five transgenic lines of B. juncea expressing PiD6. SA, Stearic acid; OA, oleic acid.
As compared with the reduction of LA in transgenics, the decrease in linolenic acid in transgenics is less dramatic but still significant. In the untransformed wild type, linolenic acid accounts for more than 10% of the total fatty acids in seeds, whereas in transgenics, the level was reduced to less than 5%.
The results are understandable. The two dramatically reduced fatty acids in transgenic seeds are the substrates of the Δ6 desaturase, and the reduction is the cost for producing two corresponding Δ6-desaturated fatty acids.
Distribution of GLA in Lipid Classes and Position of TAG
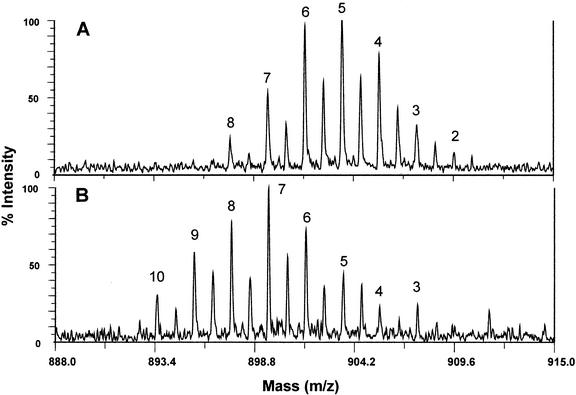
Analysis of the lipid classes of transgenic seed showed that the majority of the GLA and other new Δ6 products were incorporated almost quantitatively and exclusively into the TAGs with trace amounts found in the some of the other neutral lipids (Table I). This increase in the total lipid unsaturation can be seen in the comparison of the molecular weights of the TAGs in the transgenic and non-transgenic oil. It has been previously shown that matrix-assisted laser desorption/ionization (MALDI)-MS can be used to qualitatively and quantitatively analyze lipid components in oil seeds (Asbury et al., 1999). Figure 7 shows the shift of the molecular weights of the major TAGs down by 2 to 4 D, which indicates an additional one or two double bonds in the TAGs. The most prominent peaks in the wild-type oil correspond to five to six carbon-carbon double bonds, whereas in the transgenic oil, this shifts to seven to eight and, in contrast to wild-type oil, the transgenic oil shows significant peaks (>7% of total TAGs; data not shown) corresponding to nine and 10 C-C double bonds per molecule.
Table I.
Distribution of GLA and total lipids by lipid class (wt %) in B. juncea expressing PiD6
| Polar Lipids | Free Fatty Acids | MAGs | Diacylglycerols | TAGs | |
|---|---|---|---|---|---|
| % | |||||
| GLA | ND | 0.28 | ND | 0.14 | 98.50 |
| Total fatty acid | 0.16 | 0.67 | 0.10 | 0.23 | 97 |
ND, Not detected.
Figure 7.
MALDI mass spectrum of B. juncea seed oil TAGs containing 18-carbon fatty acids. Peaks represent M–H+Na ions and are labeled to indicate the number of unsaturations present in the molecule. The B. juncea expressing PiD6 (B) contains TAGs with higher levels of unsaturation than the corresponding wild-type B. juncea (A) TAGs.
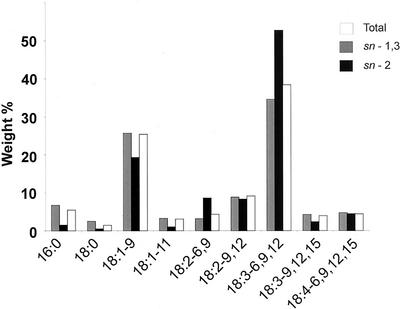
Treatment of the TAG fraction with lipase and subsequent analysis of the fatty acids present in the sn-2 monoacylglycerols (MAG) produced (Fig. 8) shows that, as expected, GLA is more abundant in the sn-2 position of the TAG than the average for the sn-1 and sn-3 positions (Stymne and Stobart, 1987). FAME analysis of the MAG showed that 52.6% (wt %) was GLA. When compared with the 38.4% in total TAGs, this indicates an average of 31.3% of the fatty acids at each of sn-1 and sn-3 positions (Christie, 1982). GC analysis of the released fatty acids in the reaction showed 34% GLA, which compares closely with the calculated value of 31.3%.
Figure 8.
Comparison of the relative wt % of fatty acids for total TAG, sn-2 position, and average of the sn-1 and sn-3 positions in TAGs of B. juncea expressing PiD6.
DISCUSSION
In this report, we describe isolation of a gene encoding Δ6 desaturase from P. irregulare. Expression of the gene in B. juncea resulted in production of a high level of Δ6-desaturated fatty acids, especially GLA in the seeds. At present, the predominant sources of GLA are oils from plants such as evening primrose (Oenothera biennis), borage, and black currant (Ribes nigrum), and from microorganisms such as Mortierella spp., Mucor spp., and cyanobacteria (Phillips and Huang, 1996). However, these GLA sources are not ideal for dietary supplementation due to large fluctuations in availability and extraction costs.
Transgenic plants have long been considered as an efficient way to produce biological compounds. The cost effectiveness of production in genetically modified plants has attracted tremendous scientific and industrial effort directed toward production of various compounds (Ohlrogge and Browse, 1998; Somerville and Bonetta, 2001). Thus, use of oilseed crops to produce the Δ6-desaturated fatty acids is economically attractive. Oilseed crops have high yield and oil content compared with the traditional GLA-producing species. For instance, with common cultivation practice in southern Saskatchewan, Canada, the yield of borage is around 70 kg of seeds per acre, the oil content is in a range of 30% to 33%, and GLA content is around 23% of the total fatty acids in seeds. The yield of B. juncea is around 600 kg of seeds per acre, and the oil content is in a range of 40% to 45% (B. McCann and D. Potts, personal communication). In the transgenic seeds (see Fig. 5), the GLA content is around 25% to 40%. The efficiency of GLA production in transgenic B. juncea mustard is obviously advantageous over the traditional borage system.
Because of the potential efficiency of transgenic production of GLA in crops, there have been several previous attempts to express Δ6 desaturases in plants (Knutzon et al., 1999; Sayanova et al., 1999a; Qiu et al., 2002). Expression of a borage Δ6 desaturase under the control of 35S promoter in tobacco and flax resulted in a high-level accumulation of GLA in vegetative tissue (more than 20% of the total fatty acids), but the level in seeds was low (less than 2% of the total fatty acids; Sayanova et al., 1999a; Qiu et al., 2002). Expression of a borage Δ6 desaturase under the control of napin promoter in B. juncea resulted in a moderate production of GLA (3%–9%) in mature seeds (Qiu et al., 2002). However, expression of a Δ6 desaturase along with a Δ12 desaturase from M. alpina under the control of the napin promoter resulted in production of up to 43% of GLA in seeds of B. napus (Knutzon et al., 1999; Palombo et al., 2000; Liu et al., 2001). The high-level production of GLA accompanied accumulation of only a small amount of SDA, no 18:2(6, 9) in the seeds. Three quarters of the GLA produced was located at sn-1 and sn-3 positions of the TAG.
Our experiments demonstrate that the introduction of a single gene encoding Δ6 desaturase from P. irregulare into B. juncea results in the accumulation of up to 40% GLA in seeds. GLA is almost exclusively incorporated into TAG (98.5%), and within the TAGs, the GLA is more abundant at the sn-2 position. It is not clear why this preference for the sn-2 position in B. juncea differs from that of B. napus. In the B. juncea system described, along with GLA, a small amount of 18:2(6, 9) also accumulates. In biotechnological applications in which this diene may be undesirable, it may be possible to reduce or eliminate it by co-expression of a Δ12 desaturase (Liu et al., 2001), thereby, reducing the level of its oleate precursor. Our results suggest that Δ6 desaturases isolated from oleaginous fungi may not be tightly regulated, as are plant counterparts in oilseed crops, or they are simply more active than those of plant sources.
MATERIALS AND METHODS
Strains and Culture Condition
Pythium irregulare 10951 obtained from the American Type Culture Collection (Manassas, VA) was grown at 25°C for 6 d in a liquid medium consisting of 3 g L−1 yeast (Saccharomyces cerevisiae) extract, 3 g L−1 malt extract, 5 g L−1 peptone, 10 g L−1 Glc, 0.68 g L−1 K2HPO4, pH 6.0, and 1 n HCl (Stinson et al., 1991). Cells were harvested by filtration and washed with distilled water three times. The dried mass was frozen in liquid nitrogen, ground with mortal and pestle into a fine powder, and stored at −70°C.
DNA Cloning
Single-stranded cDNA was synthesized from total RNA using First-Strand cDNA Synthesis kit (Amersham-Pharmacia Biotech, Uppsala) and was used as a template for PCR amplification with degenerate primers (forward; GCXA/GAXGAXCAC/TCCXGGXGG; and reverse, ATXTG/TXGGA/GAAXAG/AA/GTGA/GTG; Qiu et al., 2001a). PCR amplification was run on a DNA thermal cycler (PerkinElmer Life Sciences, Boston, MA) using a program of 1 min at 94°C, 1.5 min at 55°C, and 2 min at 72°C for 35 cycles followed by extension for 10 min at 72°C. The amplified products were separated on a 1.0% (w/v) agarose gel, and fragments from 800 to 1,000 bp were isolated and purified using a kit (Qiaex II gel purification, Qiagen USA, Valencia, CA) and were subsequently cloned into the TA cloning vector pCR 2.1 (Invitrogen, Carlsbad, CA). The cloned inserts were then sequenced by PRISM DyeDeoxy Terminator Cycle Sequencing System (PE-Applied Biosystems, Foster City, CA).
The mRNA was extracted from the total RNA by using Dynabeads oligo(dT)25 (Dynal Biotech, Lake Success, NY). The cDNA library was constructed using ZAP-cDNA Gigapack III Gold Cloning Kit (Stratagene, La Jolla, CA) and screened according to standard methods (Ausubel et al., 1995).
Expression of PiD6 in Yeast
The ORF of Pid6 was amplified by PCR using the high-fidelity Pfu polymerase (Stratagene). After initial amplification, normal Taq polymerase was added to the reaction, and it was then incubated at 72°C for another 10 min to facilitate the TA cloning (pCR 2.1, Invitrogen). Having confirmed that the PCR products were identical to the original cDNAs by sequencing, the fragments were then released by a BamHI-EcoRI double digestion and inserted into the yeast expression vector pYES2 (Invitrogen). The following yeast transformation and growth were as described by our previous work (Qiu et al., 2001b).
Transformation of B. juncea
The hypocotyls of 5- to 6-d-old seedlings of B. juncea 1424, a zero-erucic acid breeding line, were used as explants for inoculation with Agrobacterium tumefaciens GV3130::pMP90 that hosts a binary vector with the full-length PiD6 cDNA under the control of the oilseed (Brassica napus) napin promoter (−1,102 to +43; Ericson et al., 1986). The B. juncea transformation was according to Radke et al. (1992).
Fatty Acid Analysis
Total fatty acids were extracted from yeast cultures and plant seeds and were analyzed as FAMEs by GC according to Qiu et al. (2001b). For double-bond position analysis, FAMEs were saponified and derivatized with diethylamine (Nilsson and Liljenberg, 1991).
Lipid Class Analysis
Total lipids were extracted by grinding the individual seeds with a polytron homogenizer (Kinematica, Basel) in 2 mL of a mixture of CHCl3:isopropanol (2:1, v/v) containing internal standards and butylated hydroxytoluene as an antioxidant. The slurry was filtered through a glass fiber filter and eluted with CH2Cl2. A small aliquot was taken for total FAME analysis. Lipid extracts from several seeds that were determined to be high in GLA (approximately 40%) and a wild type were spotted in separate lanes along with standard lanes and developed on a silica gel 60 thin-layer chromatography plate (Merck, Rahway, NJ) and developed with a solvent mixture of hexane:diethyl ether:acetic acid (70:30:1, v/v). TAGs, diacylglycerols, MAG, free fatty acids, and polar lipids were isolated separately by scraping the appropriate areas from thin-layer chromatography plates. The lipids were eluted from the silica with 4 mL of CH2Cl2, except for polar lipids, which were eluted with acidic Bligh and Dyer (1959) solution. The lipid fractions were dried under N2 and transmethylated with 2 mL of 1% (v/v) H2SO4 in methanol at 50°C for 30 min. The mixture was cooled, 2 mL of H2O was added, and the FAMEs were extracted into hexane for GC analysis.
TAG Positional Analysis
For TAG positional analysis, lipid extracts high in GLA (as determined by GC of FAME) were pooled and applied to a 1-mL silica gel column and eluted with 3 mL of CH2Cl2 to yield the neutral lipid fraction, which was predominantly TAG. A portion of the combined eluate was dried under N2 and transmethylated to FAMEs with 2 mL of 1% (v/v) H2SO4 in CH3OH for GC analysis. The remainder was combined and subjected to a pancreatic lipase hydrolysis as described by Christie (1982) for positional analysis.
Mass spectra of TAGs were obtained from MALDI-TOF Mass Spectrometer:Voyager DE STR (PE-Applied Biosystems) equipped with a nitrogen laser 337-nm, 3-ns pulse with an average of 50 shots per spectrum acquired. Data were acquired in reflectron mode, and the M–H+Na ions were observed for the triglycerides. The matrix used was 0.5 μL of 2,5-dihydroxybenzoic acid (70 mg mL−1 in 90:10 [v/v] acetone:water; Sigma, St. Louis) spotted on plate and air-dried. The samples were dissolved in chloroform, and 0.5 μL of sample was placed on top of dried matrix film and air-dried. The instrument was calibrated using a one-point calibration with the M–H+Na ion for triolein (907.7731 D).
ACKNOWLEDGMENTS
We thank Dr. Derek Potts for providing B. juncea germplasm, Stephen Ambrose for performing GC-MS analysis, and Doug Olson for performing MALDI-MS analysis.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001495.
LITERATURE CITED
- Aki T, Shimada Y, Inagaki K, Higashimoto H, Kawamoto S, Shigeta S, Ono K, Suzuki O. Molecular cloning and functional characterization of rat delta-6 fatty acid desaturase. Biochem Biophys Res Commun. 1999;255:575–579. doi: 10.1006/bbrc.1999.0235. [DOI] [PubMed] [Google Scholar]
- Asbury GR, Al-Saad K, Siems WF. Analysis of triacylglycerols and whole oils by matrix-assisted laser desorption/ionization time of flight mass spectrometry. J Am Soc Mass Spectrom. 1999;10:983–991. [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, Albrght LM, Coen DM, Varki A. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1995. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cho HP, Takamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian Δ6 desaturase. J Biol Chem. 1999;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- Christie WW. Lipid Analysis. Ed 2. Toronto: Pergamon Press; 1982. [Google Scholar]
- Ericson ML, Rodin J, Lenman M, Glimelius K, Josefsson LG, Rask L. Structure of the rapeseed 1.7 S storage protein, napin, and its precursor. J Biol Chem. 1986;261:14576–14581. [PubMed] [Google Scholar]
- Girke T, Schmidt H, Zahringer U, Reski R, Heinz E. Identification of a novel Δ6-acyl-group desaturase by targeted gene disruption in Physcomitrella patens. Plant J. 1998;15:39–48. doi: 10.1046/j.1365-313x.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- Gunstone FD. Gamma linolenic acid-occurrence and physical and chemical properties. Prog Lipid Res. 1992;31:145–161. doi: 10.1016/0163-7827(92)90007-6. [DOI] [PubMed] [Google Scholar]
- Heinz E. Biosynthesis of polyunsaturated fatty acids. In: Moore Jr TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 33–89. [Google Scholar]
- Higgins DG, Sharp PM. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Omega-6 Essential Fatty Acids: Pathophysiology and Roles in Clinical Medicine. New York: Wiley-Liss; 1990. pp. 21–54. [Google Scholar]
- Horrobin DF. Nutritional and medical importance of gamma-linolenic acid. Prog Lipid Res. 1992;31:163–194. doi: 10.1016/0163-7827(92)90008-7. [DOI] [PubMed] [Google Scholar]
- Huang Y-S, Chaudhary S, Thurmond JM, Bobik EG, Yuan L, Chan GM, Kirchner SJ, Mukerji P, Knutzon DS. Cloning of Δ12- and Δ6-desaturases from Mortierella alpina and recombinant production of γ-linolenic acid in Saccharomyces cerevisiae. Lipids. 1999;34:649–659. doi: 10.1007/s11745-999-0410-8. [DOI] [PubMed] [Google Scholar]
- Huang Y-S, Milles DE. Gamma-Linolenic Acid: Metabolism and Its Roles in Nutrition and Medicine. Champaign, IL: AOCS Press; 1996. [Google Scholar]
- Huang Y-S, Ziboh A. Gamma-Linolenic Acid: Recent Advances in Biotechnology and Clinical Applications. Champaign, IL: AOCS Press; 2001. [Google Scholar]
- Knutzon D, Chan GM, Mukerji P, Thurmond JM, Chaudhary S, Huang Y-S. Genetic engineering of seed oil fatty acid composition. In: Altman A, Ziv M, Izhar S, editors. Plant Biotechnology and in Vitro Biology in the Twenty-First Century. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 575–578. [Google Scholar]
- Knutzon DS, Thurmond JM, Huang Y-S, Chaudhary S, Bobid EG, Chan GM, Kirchner SJ, Mukerji P. Identification of Δ5-desaturase from Mortierella alpina by heterologous expression in Baker's yeast and canola. J Biol Chem. 1998;273:29360–29366. doi: 10.1074/jbc.273.45.29360. [DOI] [PubMed] [Google Scholar]
- Liu J-W, Huang Y-S, DeMichele S, Bergana M, Bobik E, Hastilow C, Chuang L-T, Mukerji P, Knutzon D. Evaluation of the seed oils from a canola plant genetically transformed to produce high level of γ-linolenic acid. In: Huang Y-S, Ziboh A, editors. γ-Linolenic Acid: Recent Advances in Biotechnology and Clinical Applications. Champaign, IL: AOCS Press; 2001. pp. 61–71. [Google Scholar]
- Meesapyodsuk D, Reed DW, Savile CK, Buist PH, Schafer UA, Ambrose SJ, Covello PS. Substrate specificity, regioselectivity and cryptoregiochemistry of plant and animal ω-3 fatty acid desaturases. Biochem Soc Trans. 2000;28:632–635. [PubMed] [Google Scholar]
- Michaelson LV, Lazarus CM, Griffiths G, Napier JA, Stobart AK. Isolation of a Δ5-fatty acid desaturase gene from Mortierella alpina. J Biol Chem. 1998a;273:19055–19059. doi: 10.1074/jbc.273.30.19055. [DOI] [PubMed] [Google Scholar]
- Michaelson LV, Napier JA, Lazarus CM, Griffiths G, Stobart AK. Isolation of a Δ5-desaturase gene from Caenorhabditis elegans. FEBS Lett. 1998b;439:215–218. doi: 10.1016/s0014-5793(98)01385-4. [DOI] [PubMed] [Google Scholar]
- Napier JA, Hey SJ, Lacey DJ, Shewry PR. Identification of a Caenorhabditis elegans Δ6-fatty-acid-desaturase by heterologous expression in Saccharomyces cerevisiae. Biochem J. 1998;330:611–614. doi: 10.1042/bj3300611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier JA, Sayanova O, Stobart AK, Shewry PR. A new class of cytochrome b5 fusion proteins. Biochem J. 1997;328:717–720. [PMC free article] [PubMed] [Google Scholar]
- Nilsson R, Liljenberg C. The determination of double bond positions in polyunsaturated fatty acid: gas chromatography/mass spectrometry of the diethylamide derivative. Phytochem Anal. 1991;2:252–259. [Google Scholar]
- Ohlrogge J, Browse J. Manipulation of seed oil production. In: Lindsey K, editor. Transgenic Plant Research. Amsterdam: Harwood Academic Publishers; 1998. pp. 151–174. [Google Scholar]
- Palombo JD, DeMichele SJ, Liu JW, Huang YS. Comparison of growth and fatty acid metabolism in rat fed diets containing equal levels of γ-linolenic acid from high γ-linolenic acid canola or borage oil. Lipids. 2000;35:975–981. doi: 10.1007/s11745-000-0608-9. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Huang YS. Natural sources and biosynthesis of γ-linolenic acid: an overview. In: Huang YS, Milles DE, editors. γ-Linolenic Acid: Metabolism and Its Roles in Nutrition and Medicine. Champaign, IL: AOCS Press; 1996. pp. 1–13. [Google Scholar]
- Qiu X, Hong HP, Datla N, MacKenzie SL, Tayler CD, Thomas LT. Expression of borage Δ-6 desaturase in Saccharomyces cerevisiae and oilseed crops. Can J Bot. 2002;80:42–49. [Google Scholar]
- Qiu X, Hong HP, MacKenzie SL. Identification of a Δ4 fatty acid desaturase from Thraustochytrium sp. involved in biosynthesis of docosahexaenoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J Biol Chem. 2001a;276:31561–31566. doi: 10.1074/jbc.M102971200. [DOI] [PubMed] [Google Scholar]
- Qiu X, Reed DW, Hong HP, MacKenzie SL, Covello PS. Identification and analysis of a gene from Calendula officinalis encoding a fatty acid conjugase. Plant Physiol. 2001b;125:847–855. doi: 10.1104/pp.125.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke SE, Turner JC, Facciotti D. Transformation and regeneration of Brassica rapa using Agrobacterium tumefaciens. Plant Cell Rep. 1992;11:499–505. doi: 10.1007/BF00236265. [DOI] [PubMed] [Google Scholar]
- Sayanova O, Davies GM, Smith MA, Griffiths G, Stobart AK, Shewry PR, Napier JA. Accumulation of Δ6-unsaturated fatty acids in transgenic tobacco plants expressing a Δ6-desaturase from Borago officinalis. J Exp Bot. 1999a;50:1647–1652. [Google Scholar]
- Sayanova O, Shewry PR, Napier JA. Histidine-41 of the cytochrome b5 domain of the borage Δ6 fatty acid desaturase is essential for enzyme activity. Plant Physiol. 1999b;121:641–644. doi: 10.1104/pp.121.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayanova O, Smith MA, Lapinskas P, Stobart AK, Dobson G, Christie WW, Shewry PR, Napier JA. Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc Natl Acad Sci USA. 1997;94:4211–4216. doi: 10.1073/pnas.94.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J, Cahoon EB. Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- Somerville CR, Bonetta D. Plants as factories for technical materials. Plant Physiol. 2001;125:168–171. doi: 10.1104/pp.125.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling P, Lee M, Girke T, Zahringer U, Stymne S, Heinz E. A bifunctional delta-6 fatty acyl acetylenase/desaturase from the moss Ceratodon purpureus: a new member of the cytochrome b5 superfamily. Eur J Biochem. 2000;267:3801–3811. doi: 10.1046/j.1432-1327.2000.01418.x. [DOI] [PubMed] [Google Scholar]
- Sperling P, Zahringer U, Heinz E. A sphingolipid desaturase from higher plants: identification of a new cytochrome b5 fusion protein. J Biol Chem. 1998;273:28590–28596. doi: 10.1074/jbc.273.44.28590. [DOI] [PubMed] [Google Scholar]
- Stinson EE, Kwoczak R, Kurantz MJ. Effect of cultural conditions on production of eicosapentaenoic acid by Pythium irregulare. J Ind Microbiol. 1991;8:171–178. doi: 10.1007/BF01575850. [DOI] [PubMed] [Google Scholar]
- Stymne S, Stobart AK. Triacylglycerol biosynthesis. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants: Lipids. Vol. 9. New York: Academic Press; 1987. pp. 175–214. [Google Scholar]
- Wallis JG, Browse J. The Δ8-desaturase of Euglena gracilis: an alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch Biochem Biophys. 1999;365:307–316. doi: 10.1006/abbi.1999.1167. [DOI] [PubMed] [Google Scholar]